views
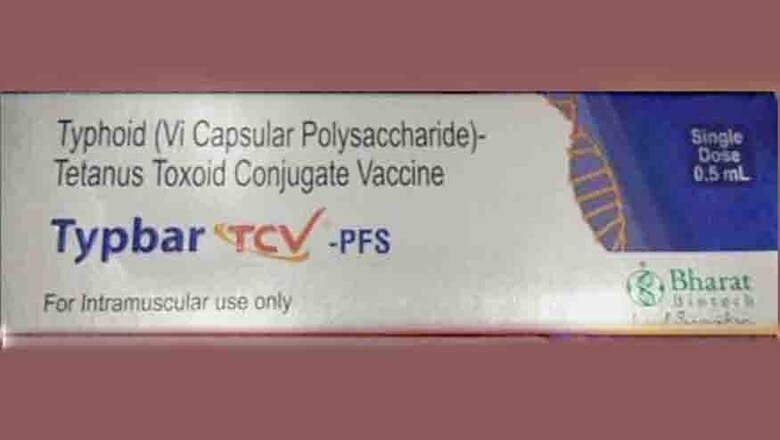
New Delhi: India's homegrown typhoid conjugate vaccine, the first of its kind worldwide, has moved one step closer to entering countries with high disease burdens of typhoid.
On Tuesday, the Geneva based GAVI, the Vaccine Alliance, gave a call for countries to apply to it for help procuring the vaccine.
Typbar-TCV has been developed by Bharat Biotech, a Hyderabad-based company. Unlike its predecessors, it can be administered to children as young as six months. Children, according to the World Health Organisation, are disproportionately affected by typhoid.
Older vaccines could only be given to children above the age of two, leaving infants vulnerable to the disease.
As News18 had reported earlier, GAVI had earmarked $85 million to fund the introduction of the vaccine to countries in need. The WHO had pre-qualified the vaccine in December 2017.
Asia has the highest disease burden for typhoid, a GAVI spokesperson told News18. Countries such as Pakistan and Bangladesh in south Asia, in particular, are badly hit by food and water-borne infectious disease.
A paper by the World Health Organisation published on March 30 noted that "global estimates of typhoid fever burden range between 11 and 21 million cases and approximately 1,28,000 to 1,61,000 deaths annually".
It added, "The majority of cases occur in South/South-East Asia, and sub-Saharan Africa."
However, the GAVI spokesperson added that the vaccine has seen interest from countries across Africa too. GAVI will now work with interested countries to plan out how many doses they need and what campaigns they need to run. The alliance ideally wants all countries to introduce the vaccine into their routine immunisation programmes, and will help plan additional campaigns to reach individuals missed in previous routine immunisation drives.
Dr. Krishna Ella, Bharat Biotech's founder and managing director, told News18 that they were already supplying doses to Pakistan, which is suffering from a typhoid outbreak that has not responded to any antibiotic.
The WHO paper touches on the growing antibiotic resistance to typhoid, saying "strains with full resistance to fluoroquinolones, such as ciprofloxacin and gatifloxacin, are now increasingly common in South Asia and are spreading in sub-Saharan Africa."
Bharat Biotech is also conducting its Phase 4 post-approval clinical trials in Bangladesh, Nepal and Malawi.
















Comments
0 comment