views
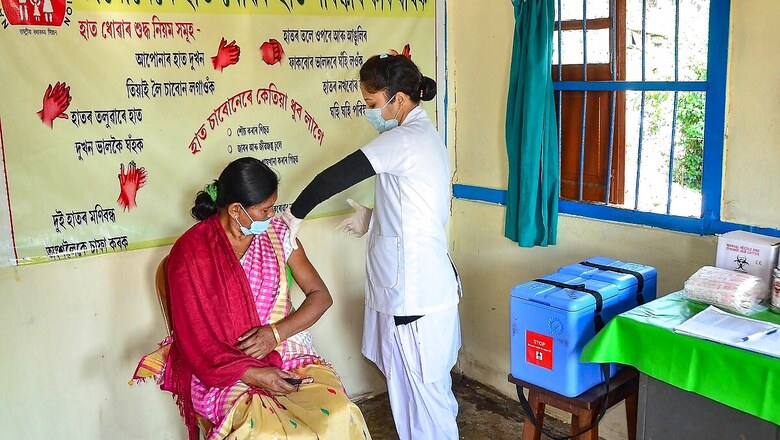
Beneficiaries who receive Bharat Biotech’s Covaxin will be paid compensation if they suffer adverse event due to the vaccine. The compensation was among the points highlighted on top of the consent form shared with the vaccination centre on Friday.
Vaccination sites at the six Mumbai central government hospitals will give Covaxin. The consent form stated that the beneficiaries will be provided care in government-designated and authorized hospitals in case of any serious side effects due to the vaccine, a Times of India report said.
Also read: Coronavirus Vaccine Drive Gathers Pace; Covishield, Covaxin Doses Reach far Corners of India
The issue of liability has been a point of contestation between the vaccine makers and the government, with the vaccine makers demanding indemnity against any mishap. However, the government’s purchase order said that the company has to be liable for any adversities.
Maharashtra officials said that the turnout today will tell whether the three-page consent form will raise concerns. Maharashtra is among the 11 states to accept Covaxin, which is under Phase 3 trials and no large efficacy data.
Also read: Coronavirus Vaccine: Bharat Biotech Signs Pact With Brazil for Supply of Covaxin
Beneficiaries of Covaxin have to sign a consent form unlike those getting the Covishield as it has been approved for restricted use in emergency situations.
According to reports, the recipient of the vaccine will also be handed fact sheet and an adverse effect reporting form where they would have to note down symptoms suffered within the first seven days. The consent form state that the vaccine has demonstrated the ability to produce antibody against coronavirus in phase 1 and phase 2 clinical trials. “However, the clinical efficacy of Covaxin is yet to be established and it is being studies in phase 3 clinical trial,” the report said.
Earlier, Bharat Biotech chairman Dr Krishna Ella speaking to News18 said India can expect interim efficacy data on its vaccine once the trials are completed. “The data is on its way. Phase III trials are going on. This sort of trial – 26,000 volunteers involved is a huge number. This has never happened. But there is not one word of appreciation for that. It is not easy to capture the efficacy now. It is easy to capture efficacy when there is a high disease burden,” Dr Ella said.
“It is easy to target Indian scientists. I had to tell this because some other company has branded my product as ‘safe like water’. Some local company in press yesterday said that safety is like water of other companies. Only three companies have done efficacy, and other vaccine is like water. I want to deny that. It hurts us as scientists; we work 24 hours and don’t deserve this type of bashing from people,” he responded to the question on the effectiveness of the vaccine.
Read all the Latest News, Breaking News and Coronavirus News here
















Comments
0 comment