
views
X
Research source
However, if handled safely with the right equipment, acids need not be feared. Use acids confidently by learning how to protect yourself, how to use the proper lab equipment, and what to do in the event of spills and other emergencies.
Protecting Yourself

Wear a lab coat or apron. Any time you work with acids, it is important to wear a lab coat or lab apron. Be sure that the sleeves cover your wrists and that it is buttoned all the way up. You will also want to make sure you meet certain safety requirements with what you wear in general. For example, you should wear closed-toe shoes, long pants, and keep your hair pulled back.

Protect your eyes with safety goggles. It is also crucial for you to protect your eyes when working with acids. Use a pair of large safety goggles that cover your eyes, both in the front and on both sides. Safety goggles often come in multiple sizes and sometimes have an adjustable strap in the back. Be sure to select goggles that fit properly, and/or make adjustments for a snug fit.

Use acid-resistant gloves. In order to properly handle acids, you will also need protective covering on your hands. Wear a pair of gloves, made from an acid-resistant material, such as nitrile or butyl. Buy these gloves at a medical supply store, cleaning store, or online.
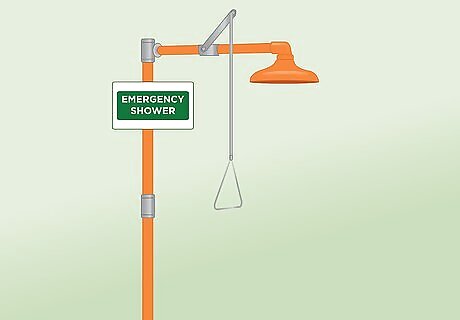
Locate emergency showers, eyewash stations, and lab spill kit. Your lab should be equipped with emergency showers, emergency eyewash stations, and at least one lab spill kit. Familiarize yourself with the locations of these stations and kits before you begin your work with acids. If your lab does not contain these stations or kits, discontinue your work in that space, as it is not safe for handling acids.

Educate yourself on what to do in the event of a spill. When a chemical or acid spill occurs, you must act quickly without panicking. You will be better able to accomplish this task if you are well educated on what to do. If you spill acid, spread sodium bicarbonate (baking soda) on the spill first to neutralize the acid. Clean it up with a paper towel afterwards and dispose of the towel. If the acid touches your skin, wash it thoroughly with water. Do not put sodium bicarbonate on your skin.
Using Proper Lab Equipment

Use acid-compatible containers. There are a wide variety of materials used for laboratory containers (such as PVC, LDPE, PP, and many others). Different acids will have divergent effects on these materials. As such, it is crucial to determine which material will be best suited for storing and transferring the acid (or acids) you will be using. For example, chromic acid (at 10%-50%) can be safely stored in LDPE. Acetic acid (50%), however, should be kept in PP. It is important to reference a chemical compatibility chart to determine the best material for your containers.

Check containers for any damages or leaks. In addition to selecting the correct material for your containers, it is very important to ensure that your containers are intact. Carefully inspect each container for leaks, cracks, or other damage before use. If you determine that a container is unusable, be sure to dispose of it according to the guidelines of your lab (Often, there will specific disposal bins for various materials.)
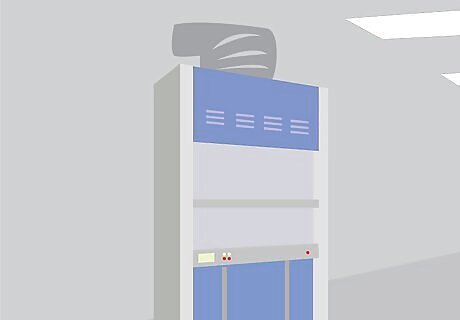
Utilize a fume hood. An excellent safety measure is to conduct all operations involving acid underneath a fume hood. It is best to work at least six inches inside the fume hood, to ensure the highest level of contaminant capture.

Use the right size container for the job. In addition to selecting a container of the proper material, it is safest to use a container of the correct size. Use of a container that is too large or too small can result in spills, leaks, or other hazards.

Store acids in a dedicated “corrosives cabinet.” For your own safety and the safety of your lab, it is a good idea to store all acids in a dedicated “corrosives cabinet” or “acid cabinet.” For the most part, you will want to avoid storing acids alongside bases. Always label acids before storing them. Consider using a color-coded cap system (acetic acid = brown, phosphoric acid = white, hydrochloric acid = blue).
Preparing Acid Solutions

Consult the MSDS. Always review the Material Safety Data Sheet (MSDS) before using any hazardous material in the laboratory. This guide will provide you with any necessary warnings, proper protocol, and important information about the acid (or other material) you are about to work with.
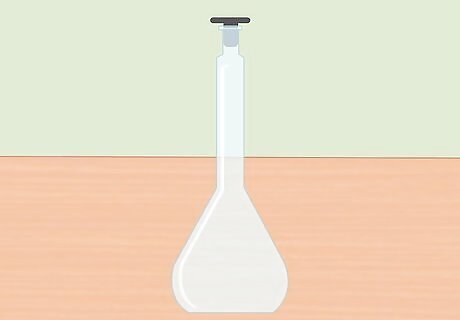
Use a volumetric flask. Before you begin preparing your solution, use a volumetric flask to measure your concentrated acid and distilled water. Be sure to use a flask made of borosilicate (or Pyrex), as this material will be acid-safe.
Begin with 2/3 of your water. To prepare an acid solution, begin by measuring out 2/3 the required amount of distilled water. Add the appropriate amount of acid to this, and stir to combine.

Add acid to water. When preparing solutions, you should always add acid to water, never the other way around. You should never add water to concentrated acid! This can generate acidic steam, and as a result, can very easily cause spills, accidents, and/or injuries.
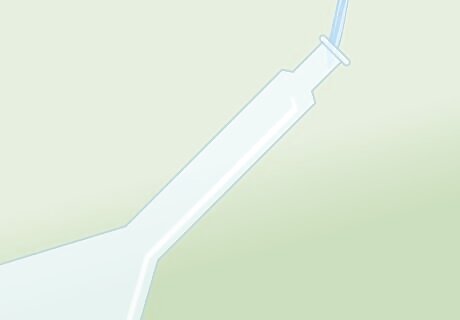
Add remaining water. Once your solution has cooled to room temperature, add water to dilute the solution to proper volume. (This will be the remaining 1/3 of the required distilled water).
Handling Emergencies

Utilize emergency showers or eyewash stations. If you should be exposed to acids, the very first thing you should do is proceed to the emergency showers (if your body was exposed) or the eyewash station (if the acid made contact with your eyes). Flush the contacted area with water for at least 15 minutes.
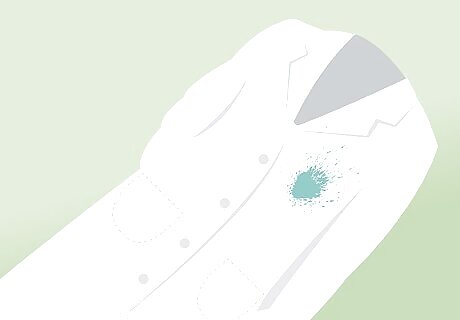
Remove all contaminated clothing in the shower. If your body and clothing have been exposed to acid, remove any and all contaminated clothing while you are in the emergency shower. You can dispose of this contaminated apparel later.
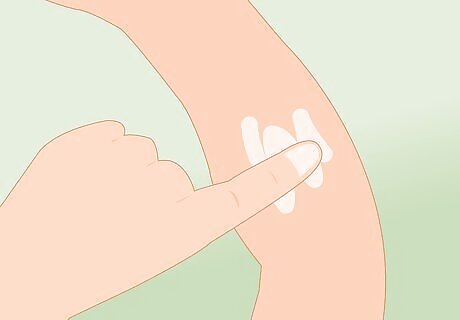
Apply calcium gluconate gel. If the spill contains hydrofluoric acid (HF) and it has made contact with your skin, you will need to apply a calcium gluconate gel right away. This will help to neutralize the hydrofluoric acid, stop the burn, and provide you with some relief.

Assess the spill. Very quickly, you will need to decide if this is a spill that can be handled by the laboratory staff, or one that requires immediate, large-scale evacuation. This will vary slightly by the capacity of your laboratory and staff. Make sure you understand what types of spills fall under which category. When an emergency occurs, quickly decide what needs to be done.

Evacuate the area. In the event of an acid spill, the laboratory will need to be evacuated. In some severe cases, you may need to evacuate the whole building. After the most acute injuries have been addressed, make sure that everyone is removed from the area. If you are on a university campus, you should also notify campus police.

Seek medical attention. Depending on the severity of your injuries, it may be necessary to seek medical attention. If you have sustained burns, injuries to the eyes, or have inhaled acid fumes, it is a good idea to visit your local doctor or hospital.



















Comments
0 comment