views
X
Research source
It’s commonly performed in experiments requiring highly diluted solutions, such as those involving concentration curves on a logarithmic scale or when you are determining the density of bacteria. Serial dilutions are used extensively in experimental sciences like biochemistry, microbiology, pharmacology and physics.
Performing a Basic Dilution

Determine the proper dilution liquid. The liquid that you will be diluting your substance in is very important. Many solutions will be diluted in distilled water, but this is not always the case. If you are diluting bacteria or other cells, you will likely want to dilute in culture media. The liquid you choose will be used for every serial dilution. If you’re unsure what liquid to use, ask for help or check online to see if other people have performed a similar dilution.
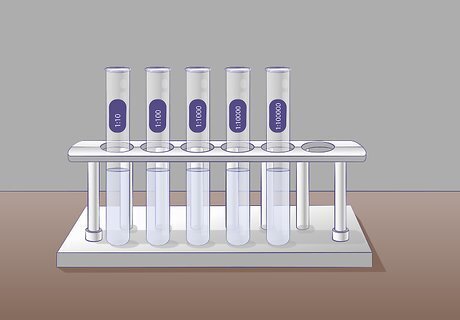
Prepare several test tubes with 9 mL of dilution liquid. These tubes will serve as your dilution blanks. You will be adding your undiluted sample to the first tube and then serially diluting into the following tubes. It’s helpful to label all of your tubes before you begin so you don’t get confused once you begin with the dilutions. Each tube will be a 10-fold dilution starting from the undiluted tube. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Determine the number of dilutions you need to do beforehand so you don’t waste tubes or diluting liquid.
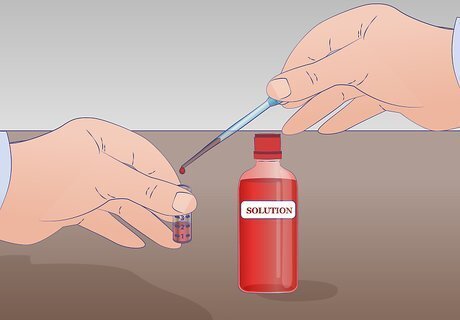
Prepare a test tube with at least 2 mL of your undiluted solution. The minimum amount needed to perform this serial dilution is 1 mL of undiluted solution. If you only have 1 mL you will not have any remaining undiluted solution. Label this tube US for undiluted solution. Thoroughly mix your solution before starting any dilutions.
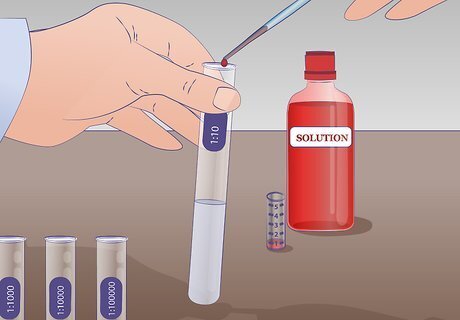
Perform the first dilution. Draw 1 mL of undiluted solution from test tube US with a pipette and transfer it to the test tube labeled 1:10 containing 9 mL of the dilution liquid and mix thoroughly. There is now 1mL of the undiluted solution in 9 mL of the dilution liquid. The solution, therefore, has been diluted by a factor of 10.
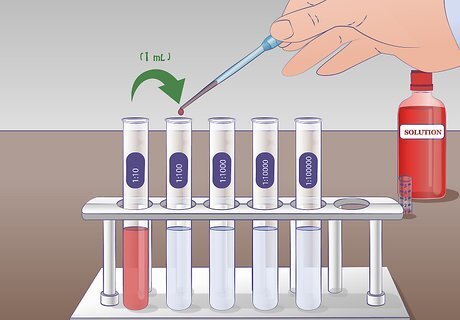
Perform the second dilution. For the second serial dilution, you will take 1 mL of solution from tube 1:10 and add it to the 9 mL of dilution liquid in the tube 1:100. Thoroughly mix tube 1:10 before adding to the next tube. Again, mix the tube 1:100 following dilution. The solution from test tube 1:10 has been diluted 10-fold into test tube 1:100.
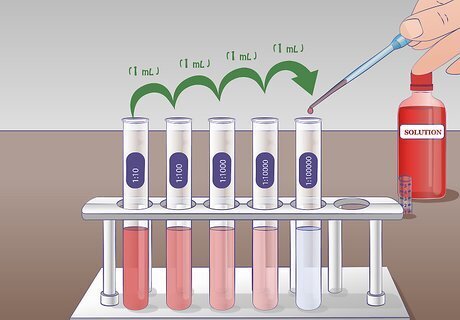
Extend this procedure to perform longer serial dilutions. This process may be repeated as many times as necessary to achieve the desired solution. In an experiment involving concentration curves, you can use a serial dilution to create a series of solutions with dilutions of 1, 1:10, 1:100, 1:1,000.
Calculating Final Dilution Factor and Concentration
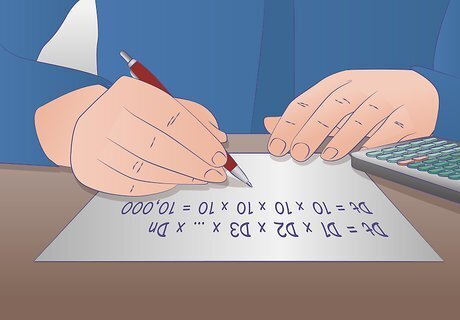
Calculate the final dilution ratio in a serial dilution. The total dilution ratio can be determined by multiplying the dilution factor of each step leading up to the final step. This can be mathematically illustrated with the equation Dt = D1 x D2 x D3 x … x Dn where Dt is the total dilution factor and Dn is the dilution ratio. For example, let’s say you did a 1:10 dilution of your liquid 4 times. Plug your dilution factor into the equation: Dt = 10 x 10 x 10 x 10 = 10,000 The final dilution factor of the fourth tube in your serial dilution is 1:10,000. The concentration of your substance is now 10,000 times less than the original undiluted solution.

Determine the concentration of the solution following dilution. To determine the final concentration of your solution following serial dilution you will need to know your starting concentration. The equation is Cfinal = Cinitial/D where Cfinal is the ending concentration of the diluted solution, Cinitial is the starting concentration of the original solution and D is the dilution ratio previously determined. For example: If you started with a solution of cells with a concentration of 1,000,000 cells per mL and your dilution ratio is 1,000, what is the final concentration of your diluted sample? Using the equation: Cfinal = Cinitial/D Cfinal = 1,000,000/1,000 Cfinal = 1,000 cells per mL. The clear explanations helped me finally grasp the method. "Prepping for a seminar, I was totally confused by serial dilutions. But this article lays out the process in simple language and easy steps. Now I really understand this important technique." - Moza S. Following the guide, I nailed the dilutions. "As a lab assistant, I didn't initially get how to do serial dilutions. But after finding this wikiHow, the detailed instructions and photos walked me through it seamlessly. I was finally able to properly execute the dilutions for our experiment." - Anthony H. The article drove home key principles from lectures. "After learning about dilutions in class, seeing real examples here made it all click. Going through sample calculations and concentration determinations reinforced the core concepts. This practical knowledge will be clutch as I start hands-on work." - Lidia N. As an educator, the article helps demonstrate the technique. "As a microbiology professor, I aim to teach students essential lab skills. This piece perfectly illustrates the dilution process and its use in research. Now I can better equip future scientists to apply this method themselves." - Katan A. The step-by-step guide eliminated my confusion. "When my teacher first covered dilutions, I didn't get the calculations at all. Walking through examples of computing ratios and final concentrations answered all my questions. Now, I totally grasp the math behind this important technique." - Leslie G. We want to hear from you! Advice from our readers makes our articles better. If you have a story you’d like to share, tell us here.
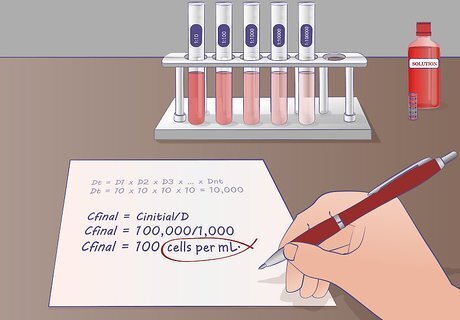
Confirm that all units match. When performing any calculation, you want to make sure that your units always match at the end. If you started with cells per mL make sure you are ending with cells per mL. If your starting concentration is parts per million (ppm), then your final concentration must be ppm.



















Comments
0 comment