views
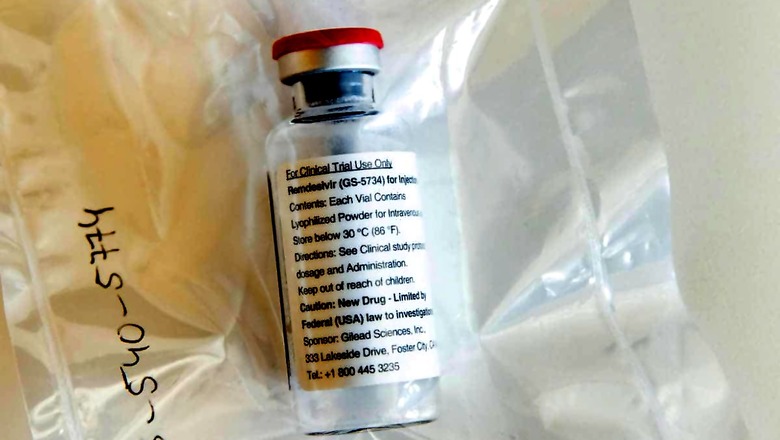
As India’s Covid-19 count breaches the 2 lakh daily count, there is panic as Remdesivir, the anti-viral injection being used to treat severe Covid-19 cases has run out.
Social media is awash with SOS messages by relatives and friends.

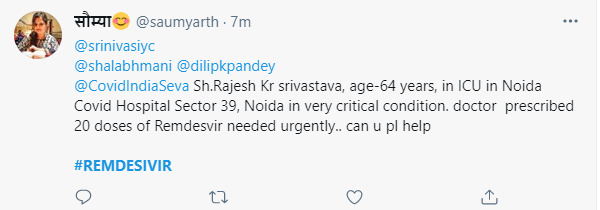
A thriving black market has cropped up. There are long queues outside stores of Remdesivir distributors. Websites like Dr Reddy’s tracking the availability of the drug say there is no stock available.

Q: What does the government say?
The government says the drug is “available in plentiful”. The panic has been induced by the “irrational use of the drug” by doctors.
Dr VK Paul, head of India’s Covid–19 task force in a press briefing, said: “Remdesivir is an investigational drug, whose use in home settings is unethical”.
According to protocol, it can only be used on hospitalised patients who are on oxygen. He has requested doctors to use Remdesivir rationally and judiciously.
Q: What do doctors say?
Dr S Chatterjee, an Internal Medicine Specialist at the Indraprastha Apollo Hospital, says it is unfair to blame doctors for the Remdesivir shortage. Clinical experience over the last one year shows that if Remdesivir is used in the “right patients, at the right time, it has a beneficial impact”.
Right patient means the elderly and those with comorbidities and right time means early enough in the hospitalisation. This use is not “reckless”, it is an attempt to save a patient’s life. Given that there are limited drugs that can help fight the virus, doctors are using whatever they have in their arsenal. “Not just Remdesivir, even Favipiravir, the other antiviral option to fight Covid-19 has run out in Delhi.”
The shortage, he says, is because of “manufacturers slowing down production , hoarding and exports.”
Q: Why did pharma companies slow down production?
There are seven companies that make Remdesivir in India like Zydus Cadila, Hetero Drugs, Cipla, Dr Reddy’s, Sun Pharma, and Jubilant Life Sciences. Together they have the capacity to produce 38.80 lakh vials per month. The government may have given the green signal to double the production, but it will take time. At the end of 2020, as Covid-19 cases dipped and the demand for Remdesivir dried up. With a result between December and February, there was almost zero production of the drug. By mid-March as the second wave became visible, there was a scaling up but the 25 raw materials needed to make the drug were not available immediately, courtesy the Covid-19 situation.
Speaking to the Economic Times, Dr Sharvil Patil, the MD of Zydus Cadila, the largest maker of the drug says that “nothing can happen overnight”. It takes 5 days to produce the drug and then it needs to undergo a 14 days sterility test. Add 3 days for transportation and you are looking at a 25 day cycle. By the end of the month the situation should ease.
Q: What has the government done?
The government has banned the export of Remdesivir injections and its active pharmaceutical ingredients. Two days ago, it also fast tracked applications to scale up production of Remdesivir. The seven pharmaceutical companies that make the drug can now double their capacity and produce 78 lakh vials together. The health ministry has also asked all manufacturers to display the details of their stockists and distributors on their websites. And four lakh vials that were meant for export have been diverted for domestic use.
In Maharashtra, where the shortage is acute, the government has mandated that the drug be provided only through collectors and area drug inspectors.
Q: Does Remdesivir work against Covid-19?
The jury is still out on how effective Remdesivir is against Covid-19. It is part of the Govt of India’s protocol to treat serious infections. It has FDA approval in the USA where studies have shown it speeds recovery in hospitalised cases. An IDSA panel says Covid-19 patients with supplemental oxygen, who are not on mechanical ventilation can be treated with five days of injections of Remdesivir, instead of 10.
But the WHO says it is not the lifesaver as it is made out to be.
Remdesivir was developed by Gilead Science in collaboration with the US’s Center for Disease Control and the US Army’s medical research institute of Infectious Diseases. It was designed as an experimental drug to combat RNA viruses that have the potential to cause global pandemics. It was ineffective against Ebola and yet was repurposed for SARS-CoV-2 (Coronavirus). Currently, it has been authorised for emergency use in 50 countries.
Q: What do courts say? (Yes, they have a view too)
The Gujarat High court hearing a suo moto case on the Covid-19 surge in the State asked the State government to put out a clear statement on the “myths of Remdesivir”. Hauling up the State administration, it blamed the State government of under reporting Covid numbers and leading to a shortage of remdesivir. When the government lawyer put the blame on doctors for prescribing it “indiscriminately”, the court shot back saying “where is the data to prove this”. The court wants the State government to clarify to the court and general public if remdesivir is effective in treating serious cases. The court as a parting shot asked the government to issue an advisory on the use of the drug and said: “I don’t know if it is a life-saving drug, but it has unnecessarily been seen as amrit.”
Q: Where should you look for Remdesivir?
Cipla is transporting Remdesivir injections directly to hospitals where a patient has been admitted to beat the black market.
Their website – www.cipla.com, helpline – 8657311088 and email – [email protected] can be contacted for help.
Dr Reddy’s website readytofightCovid.in lists all hospitals and pharmacies that stock the drug.
Their 24/7 helpline is – 1800-266-708
Q: How does it work?
Remdesivir works by blocking the virus from copying itself, veterinary microbiologist Niranjana Rajalakshmi writes in The Wire.
It essentially tricks the virus into believing that it is a building block of the viral’s genetic material.
Remdesivir is a prodrug. Which means it’s inactive at the time of injection. Once injected, the host cell metabolises it into its active form, Remdesivir triphosphate.
This is where the game begins. Remdesivir triphosphate is very similar to something the virus produces when it replicates its genome.
It is a nucleotide called adenosine triphosphate.
The Remdesivir triphosphate tricks the virus into using it into the copies of its RNA instead of adenosine triphosphate. It cleverly evades the proofreader enzyme by slotting itself into the RNA at a slightly different position from where the adenosine triphosphate is supposed to be.
This ultimately prevents the replication of the coronavirus.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.

















Comments
0 comment