views
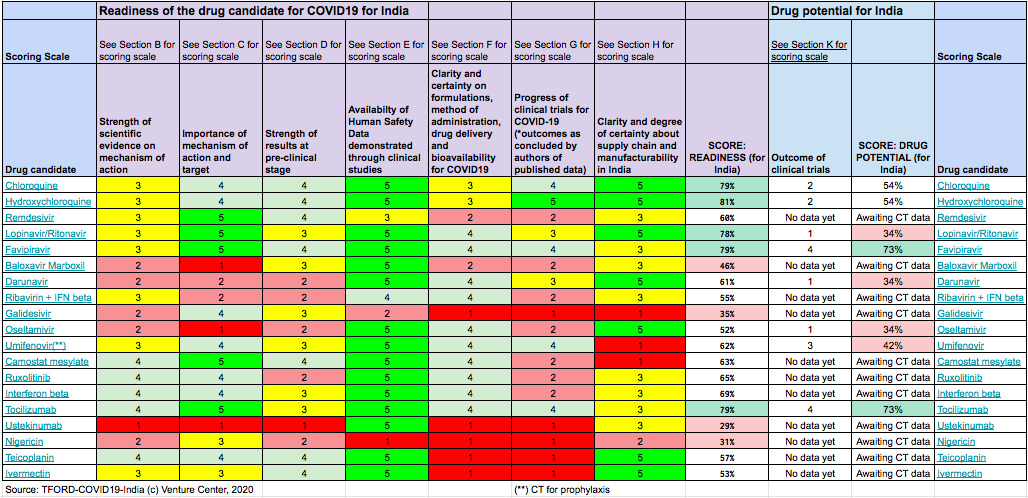
New Delhi: Antiviral drug Favipiravir and immune-modulator drug Tocilizumab are the most promising drugs in terms of their readiness for use and potential against Covid-19, the government task force for repurposing of drugs (TFORD) has said in its first such assessment.
The assessment will be dynamic and will be updated as and when fresh scientific literature and clinical trial data is available for review.
Hydroxchloroquine (HCQ), the anti-malarial drug that was recommended by Indian Council of Medical Research (ICMR) for use among health care workers as a prophylaxis (for prevention) did not score high on the ‘drug potential’ parameter even as it scored well on the readiness index, the assessment reviewed by News18.com showed.
Favipiravir is an anti-viral drug and it is approved in Japan for treating influenza. It is currently being tested in 18 clinical trials for Covid-19 and results from two studies have shown a positive outcome while data from other trials is awaited.
Tocilizumab is an immune-modulator approved for treatment of several autoimmune diseases. It is being tested in 24 clinical trials for COVID-19 worldwide and early results indicate that it can be a promising therapy for patients who are critically ill, the task force assessment said. Both these drugs have the potential to be manufactured in India, the assessment added.
The assessment on HCQ and Remdesivir will be clearer when more data is available from clinical trials underway across the globe.
The task force for repurposing of drugs was by constituted by the government’s Principal Scientific Advisor, K VijayRaghavan, for inter-disciplinary assessment of drug candidates, coordination and to support informed decision making.
The task force curated available information from scientific literature and clinical trials data for 19 drug candidates that are in various stages of development for use against Covid-19 in India. The assessment was done based on a heat map and a scoring system vetted by an inter-disciplinary advisory group to assess the readiness and potential of a drug to be used as a therapy for Covid-19 in the Indian context.
The scoring system factored in parameters such as availability and quality of scientific information from clinical trials, safety, readiness to manufacture the drugs in India and status of intellectual property. According to the task force, over 60 drugs are currently being tested in clinical trials for COVID-19 worldwide. Many of them have been approved for use in other indications and several of them are manufactured in India.
“This is a first such readiness score and potential score we have prepared based on the assessment of molecules we are examining for repurposing of drugs. The scores reflect the verified information available so far on clinical trials, efficacy, safety and readiness to manufacture the drugs,” said Dr.V.Premnath, coordinator of the task force and Director, Venture Center, science business incubator under Council of Scientific and Industrial Research.
The 19 drugs under consideration for repurposing are; Chlroquine, HCQ, Remdesivir, Lopinavir/Ritonavir, Baloxavir Marboxil, Darunavir, Ribavirin+IFN betam Galidesivir, Oseltamivir, Umifenovir, Camostat mesylate, Ruxolitinib, Interferon beta, Tocolizumab, Ustekinumab, Nigericin, Teicoplanin and Ivermectin.
When asked about HCQ, the drug India has exported to US and Brazil, Dr.Priya Nagaraj, Manager, Bioincubator, Venture Center, said, “As far as HCQ is concerned, clinical trials are ongoing outside India. There has been mixed evidence on its efficacy. Three studies have reported positive results and one has reported a negative outcome. This is a continuously evolving situation and data from more clinical studies will help in making an informed decision. We will consider factors such as quality of study design, outcomes reported by the authors to evaluate the score for each drug.”


















Comments
0 comment