views
With a vaccine still a long distance away and no sign of the coronavirus infections slowing down in the country, homegrown pharmaceutical companies have been given permission to launch generic versions of Remdesivir and Favipiravir, anti-viral drugs that have shown promise in treating Covid-19 patients.
While Glenmark Pharmaceuticals has launched favipiravir under the brand name FabiFlu for treatment of mild to moderate cases of Covid-19, Cipla and Hetero Labs have received approvals from the Drug Controller General of India to launch generic versions of Remdesivir under the brand names Cipremi and Covifor respectively.
While medical experts have cautioned against seeing these potential drugs as a ‘magic bullet’ against the deadly virus, they can be helpful in reducing the viral load as India continues to post new record highs in infections on a daily basis. Remdesivir and Favipiravir are two of the 130 under experimentation worldwide to treat Covid-19.
Covifor and Cipremi
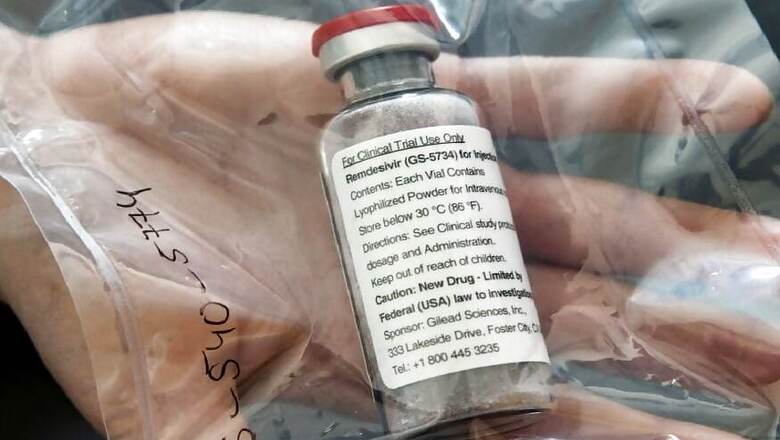
The two drugs launched by Cipla and Hetero are generic versions of Remdesivir, an antiviral drug first developed for treating Ebola in 2014. It is administered intravenously and inhibits viral replication in the body.
Last month, the US National Institutes of Allergies and Infectious Diseases had released preliminary trial results showing recovery time of Covid-19 patients given Remdesivir improved from 15 to 11 days.
The health ministry had approved the use of Remdesivir for mild cases of Covid-19 thorough an emergency authorisation route. Gilead Sciences, a United States-based company, has a patent on the drug and has given voluntary licence to Indian manufactures.
Hetero has said it would supply its version of Remdesivir at Rs 5,000- Rs 6,000 per vial, so that a five-day treatment course should not cost more than Rs 30,000 per patient. Cipla has not yet disclosed the pricing of its version.
Since Remdesivir has not been approved as treatment for Covid-19 yet and has been approved by the DCGI only for “restricted emergency use”, a patient would need to sign a consent form in case it is administered.
It is not recommended for those with severe renal impairment and high level of liver enzymes, pregnant and lactating women, and those below 12 years, the union health ministry’s document on 'Clinical Management Protocols for COVID-19' stated.
The drug, administered in the form of injection, should be given at a dose of 200 mg on day one followed by 100 mg daily for five days, it said.
Apart from Cipla and Hetero Labs, Jubilant Lifesciences and Mylan will further expand supply of the drug in India.
Fabiflu
Fabiflu will be manufactured by Mumbai-based Glenmark Pharmaceuticals as the generic version of Favipiravir, an anti-viral drug approved in Japan for treating influenza.
The oral medication is approved only for emergency restricted use in mild to moderate Covid-19 cases. The approval’s restricted use entails responsible medication use where every patient must have signed informed consent before treatment initiation.
It is currently being tested in 18 clinical trials for Covid-19 and results from two studies have shown a positive outcome, while data from other trials is awaited. Glenmark has claimed that Favipiravir shows clinical improvements of up to 88 per cent in Covid-19, with rapid reduction in viral load by four days.
The drug will be available as a prescription-based medication for Rs 103/tablet, with recommended dose being 1800 mg twice daily on day 1, followed by 800 mg twice daily up to day 14.
The Council of Scientific and Industrial Research had also done end-to-end synthesis of Favipiravir in April and another multi-centre Phase-II drug trial is now being done by it. Officials have said that the drug price can further be reduced by 20-30 per cent.
Other Potential Treatments
Tocilizumab, an immunosuppressant commonly used to treat for rheumatoid arthritis, has been used to treat more than 100 severely ill Covid-19 patients in Mumbai. A randomised control trial is also being conducted across several centres in India.
Itolizumab, a drug commonly used for the skin disorder psoriasis, rheumatoid arthritis, multiple sclerosis, and autoimmune disorders, is being trialled on Covid-19 patients in Delhi and Mumbai.
Plasma therapy has also shown some positive results for treatment of Covid-19 patients. It involves transfusion of plasma from a recovered coronavirus patient to a critical patient. The blood of a convalescent patient is rich in antibodies that are expected to help the critical patient recover.
A protocol approved by ICMR is used to select which patient is best suited for plasma therapy. Preference is given to those at risk of cytokine storm, extreme breathlessness with severe pneumonia.

















Comments
0 comment