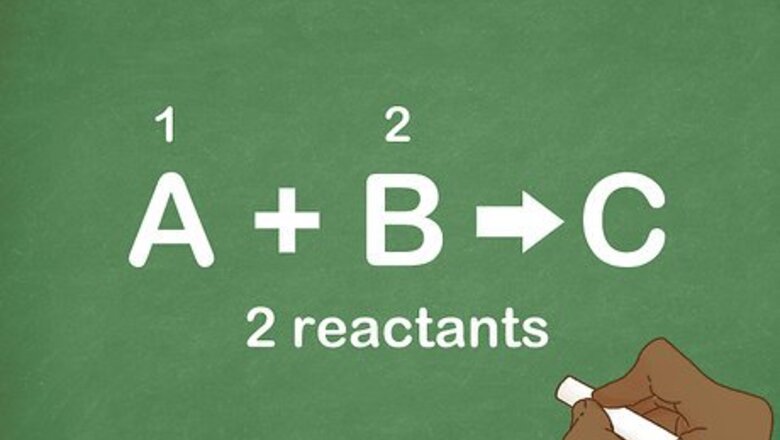
views
Identifying Combination/Synthesis Reactions
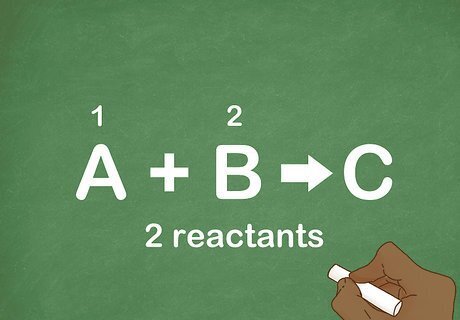
Count the number of reactants. A combination/synthesis reaction is aptly named because it is a reaction where 2 or more products combine together to form 1 new product. Remember that the reactants of an equation are always on the left side of the arrow. Many reactions have just 2 reactants, but you can have combination reactions with more than 2 reactants.
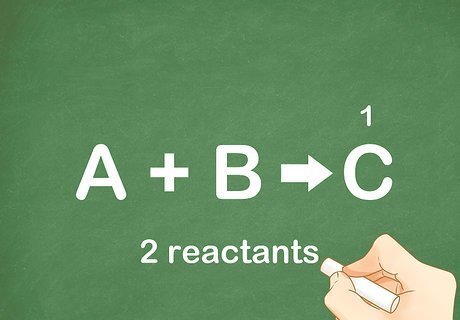
Verify that there is only 1 end product. As the name of the reaction indicates, the reactants must combine to form a new product. All products are found on the right side of the arrow. Very occasionally, there will be more than 1 product on the right side; however, most equations will only have 1 product. An example of a reaction resulting in 2 products: CO2 + H2O --> C6H12O6 + O2
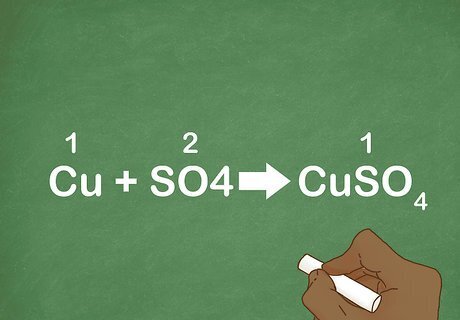
Practice with some examples. Examples are a great way to train yourself to recognize specific chemical reactions. The more examples you look at, the more likely you are to remember each type of reaction. Example 1: Cu + SO4 --> CuSO4 Example 2: CaO + CO2 --> CaCO3
Recognizing a Decomposition Reaction
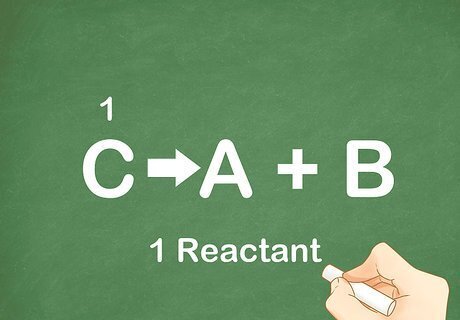
Count the number of reactants. A decomposition reaction is one where a reactant degrades or decomposes into its constituent parts. Energy, in the form of light, heat, or electricity, is usually the catalyst for the reaction. This type of reaction yields more products than reactants. Almost all basic decomposition reactions will have one reactant. The reactant is on the left side of the arrow.
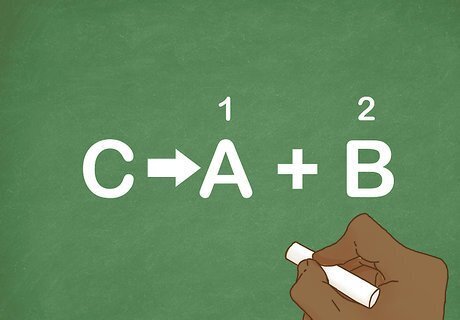
Verify that there are 2 or more end products. The reactant decomposes into multiple products. To recognize this type of reaction, just see if the equation resembles the general formula C --> A + B. Remember, the products are on the right side of the arrow. This reaction is the opposite of a combination reaction.
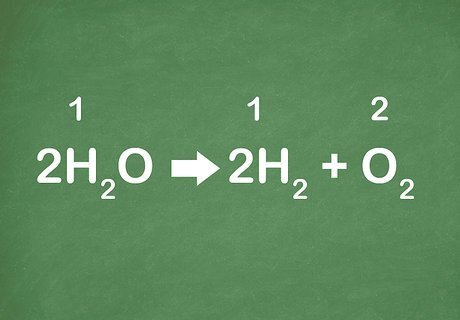
Practice with some examples. Being able to recognize equations comes with practice. The more equations you look at, the easier it will be for you to immediately realize the reaction is decomposition. Example 1: 2H2O → 2H2 + O2 Example 2: KClO3 --> KCl + O2
Identifying a Single Replacement Reaction
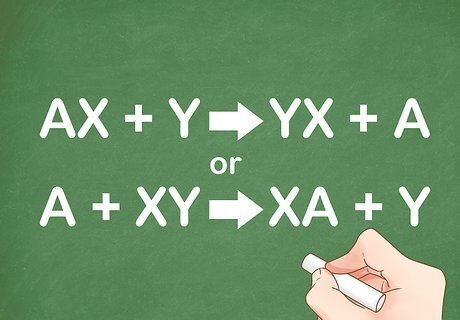
Recognize the general formula for single replacement. A single replacement reaction occurs when one element replaces another element within a compound. It generally takes the form of AX + Y --> YX + A or A + XY --> XA + Y. One reactant is always a single element and the other reactant is always a compound. In a single replacement reaction either the anion (negatively charged ion) or the cation (positively charged ion) is replaced. For example: Cu + AgNO3 --> Ag + Cu(NO3)2. In this example, copper (Cu) replaces the cation silver (Ag).
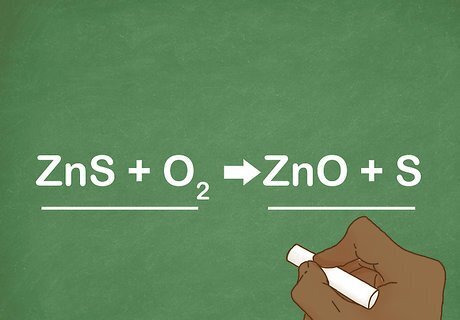
Compare the reactant and product sides. When looking at the equation, you can easily tell if a single replacement has occurred if one of the elements has switched places into the new compound. Using the general formula as guidance, you can identify the reaction. For example: ZnS + O2 --> ZnO + S
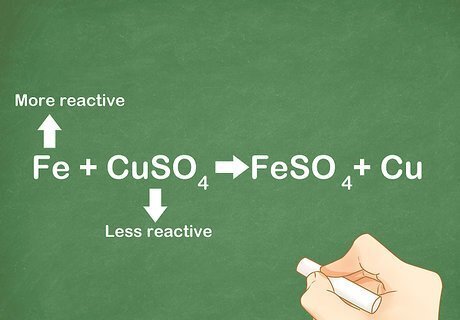
Practice with some examples. Being able to quickly recognize a single replacement reaction takes time and practice. By looking at many different types of examples, you will improve your ability to identify this reaction without looking it up. Example 1: Fe + CuSO4 → FeSO4 + Cu. Iron replaces the copper element in the compound. Example 2: Fe + HCl --> FeCl3 + H2. Iron replaces the hydrogen. Example 3: CaO + Al --> Al2O3 + Ca. Aluminum replaces the calcium.
Recognizing a Double Replacement Reaction
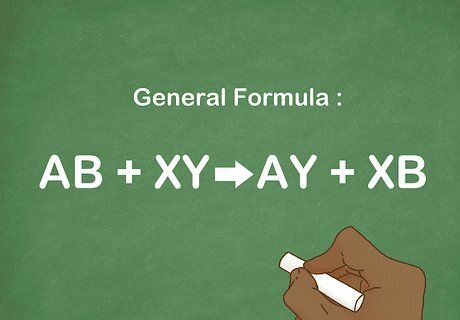
Learn the general formula for a double replacement reaction. These reactions are similar to single replacement reactions except that both components react and there are 2 replacements. The general formula is AB + XY --> AY + XB. The cations and anions from both compounds recombine to form 2 new compounds. These reactions are usually between acids and bases or metallic aqueous compounds. For example: KOH + H2SO4 --> K2SO4 + H2O.
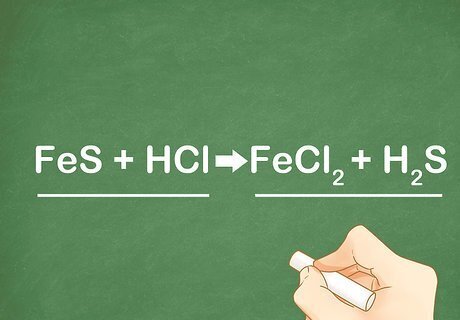
Compare the product and reactant sides. When looking at an equation you can tell it is a double replacement reaction because the outside elements will recombine to make a new compound and the inside elements will recombine to make a new compound. The inside elements will switch position because the cation is always written first. For example: FeS + HCl --> FeCl2 + H2S. The outside elements, Fe and Cl, combine to form FeCl2. The inside elements, S and H, switch positions and combine to form H2S.
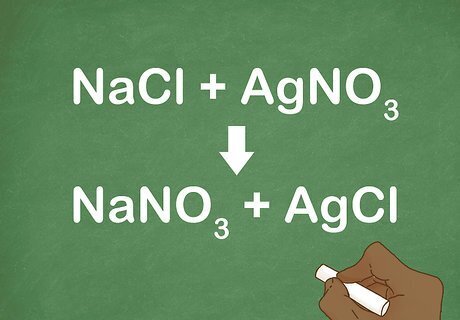
Practice with some examples. Looking at many examples of a double replacement reaction will help you recognize them when you see them on a quiz or test. The more examples you look at, the better you will be at identifying them. Example 1: NaCl + AgNO3 → NaNO3 + AgCl Example 2: H2SO4 + 2NaOH→ Na2SO4 + 2H2O
Identifying a Combustion Reaction
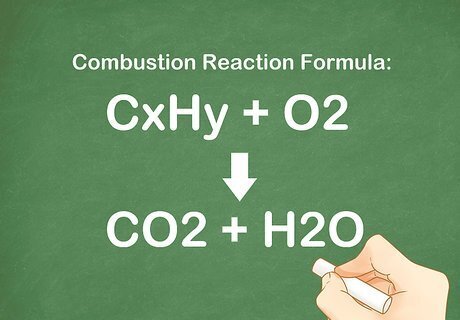
Learn the components of a combustion reaction. On a most basic level, a combustion reaction is one where oxygen gas (O2) reacts with anything to form carbon dioxide and water. Generally, the oxygen gas reacts with a compound of carbon and hydrogen. The combustion products are always CO2 and H2O. The generic equation for a combustion reaction is: CxHy + O2 --> CO2 + H2O.
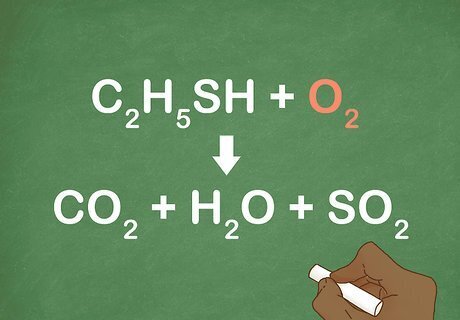
Verify that O2 is one of the reactants. The first step to identifying a combustion reaction is making sure that oxygen gas is 1 of the reactants. If there is no O2 present, then the reaction is not combustion. For example: C2H5SH + O2 --> CO2 + H2O + SO2. O2 is reacting with a carbon-hydrogen compound so this reaction is probably a combustion reaction.
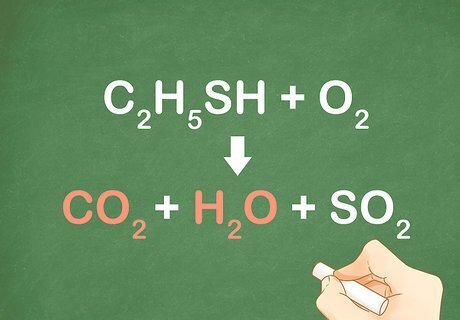
Check that the products yield CO2 and H2O. When a combustion reaction occurs, the products will almost always contain CO2 and H2O. If both carbon dioxide and water are not products of the reaction than combustion is not occurring. For example: C2H5SH + O2 --> CO2 + H2O + SO2. Because CO2 and H2O are both products, this reaction is an example of combustion.
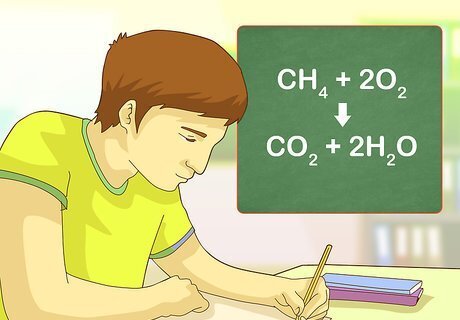
Practice with some examples. The more problems that you do, the easier it will be for you to recognize a combustion reaction when you come across it. With some practice, you'll be able to instantly identify a combustion reaction when you see one. Example 1: CH4 + 2O2 --> CO2 + 2H2O Example 2: C2H5OH + 3O2 --> 2CO2 + 3H2O
Recognizing a Reaction through Observation
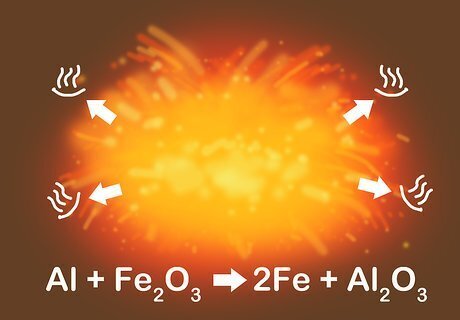
Feel for heat in exothermic reactions. Many synthesis and replacement (single and double) reactions are exothermic, meaning they release heat. Reactions that release a lot of heat, such as the thermite reaction, can be explosive. Take proper safety precautions when working with heat, such as wearing gloves and using eye protection. The thermite reaction is a single-replacement reaction between iron (III) oxide and aluminum: 3Fe302 + 4Al → 2Al203 + 6Fe
Look for the formation of precipitate. Again, in many synthesis and replacement (single and double) reactions, a precipitate will form at the bottom of the tube. A precipitate is any solid material that is insoluble in water. Sodium chloride is the white powder formed when molten sodium burns in chlorine gas.

Add heat for endothermic reactions. Most decomposition reactions are endothermic meaning you need to add heat for the reaction to occur. If heat must be added, you may be observing a decomposition reaction. An example of a decomposition reaction is mercury (II) oxide decomposing into mercury metal and oxygen gas in the presence of heat: 2 HgO (s) + heat → 2 Hg (l) + O2 (g)

Watch for light and feel for heat from combustion reactions. Combustion reactions tend to explode, forming large amounts of light and heat energy. Oftentimes, this energy is released as fire. Combustion reactions are always exothermic, meaning they release heat. Some examples of combustion reactions are: hydrogen with oxygen, phosphorus with oxygen, and magnesium with oxygen.



















Comments
0 comment