views
Naming Ionic Compounds
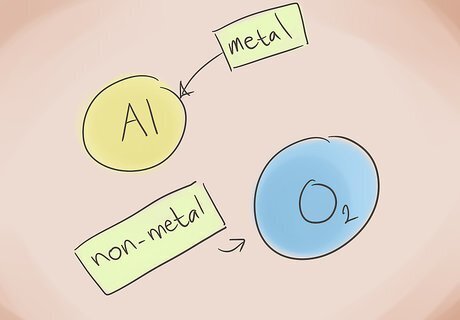
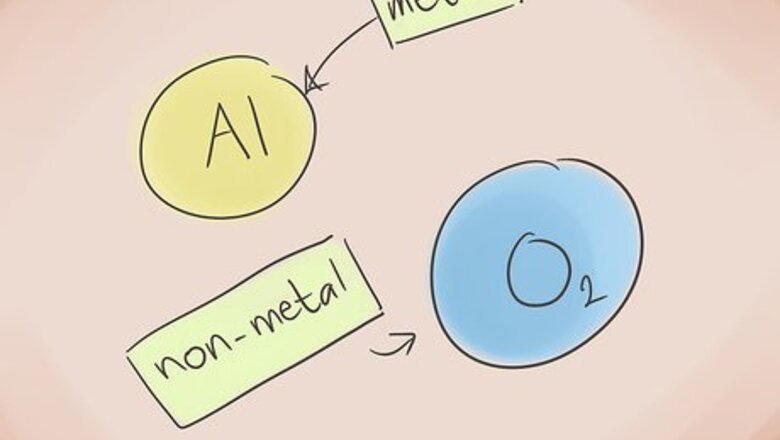
Know what makes a compound ionic. Ionic compounds contain a metal and a nonmetal. Refer to the periodic table of elements to see what categories the elements in the compound belong to.
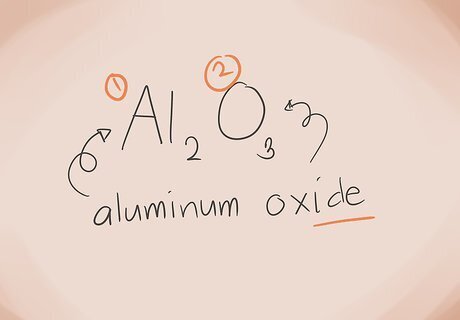
Build the name. For a two element ionic compound, the naming is simple. The first part of the name is the name of the metal element. The second part is the name of the nonmetal element, with the suffix “-ide.” Here are some examples: Al2O3. Al2 = Aluminium; O3 = Oxygen. So the name would be “aluminium oxide.” FeCl3. Fe = Iron; Cl3 = Chlorine. So the name would be "iron chloride." SnO2. Sn = Tin; O2 = Oxygen. So the name of this compound would be Tin Oxide.
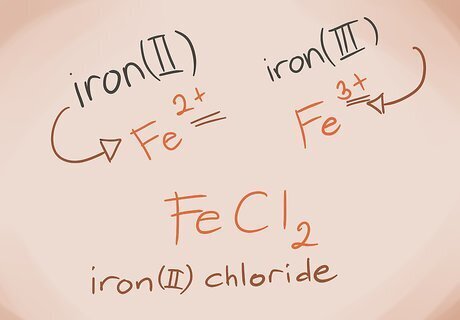
Recognize and name transition metals. Metals in the D and F blocks of the periodic table are known as transition metals. Their charge is written with a Roman numeral when writing out the compound name. This is because they can have more than one charge and make more than one compound. Example: FeCl2 and FeCl3. Fe = Iron; Cl2 = Chloride -2; Cl3 = Chloride -3. The names would be iron(II) chloride and iron(III) chloride.
Naming Polyatomic Compounds
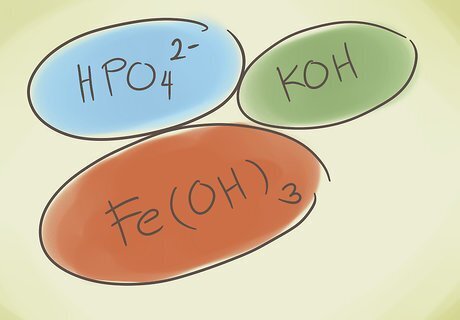
Understand what a polyatomic compound is. These compounds are built off of groups of atoms that are covalently bonded together, and the entire group has a positive or negative charge. You can do three basic things to polyatomic compounds, which will help you identify and understand these types of compounds: Add a hydrogen to the beginning of the compound. The word “hydrogen” is added to the beginning of the compound name. This reduces the negative charge by one. For example, “carbonate” CO3 becomes “hydrogen carbonate” HCO3.Name Chemical Compounds Step 4Bullet1.jpg Remove an oxygen from the compound. The charge stays the same and the ending of the compound changes from “-ate” to “–ite”. For example: NO3 to NO2 goes from “nitrate” to “nitrite.”Name Chemical Compounds Step 4Bullet2.jpg Replace the central atom with another from the same periodic group. For example, sulfate SO4 can become Selenate SeO4.Name Chemical Compounds Step 4Bullet3.jpg
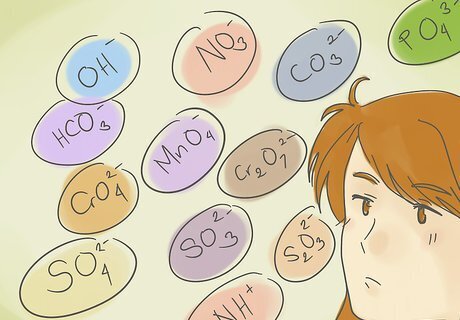
Memorize the most common ion groups. These are the basis for forming most polyatomic compounds. Listed in order of increasing negative charge, they are: Hydroxide ion: OH Nitrate ion: NO3 Hydrogen carbonate ion: HCO3 Permanganate ion: MnO4 Carbonate ion: CO3 Chromate ion: CrO4 Dichromate ion: Cr2O7 Sulfate ion: SO4 Sulphite ion: SO3 Thiosulfate ion: S2O3<2- Phosphate ion: PO4 Ammonium ion: NH4

Build compound names based off the list. Associate whatever element(s) is attached to the group ion and name accordingly. If the element comes in front of the ion group, the name of the element is simply added to the beginning of the compound name. Example: KMnO4. You should recognize the MnO4 ion as permanganate. K is potassium, so the compound is named potassium permanganate.Name Chemical Compounds Step 6Bullet1.jpg Example: NaOH. You should recognize the OH ion as hydroxide. Na is sodium, so the compound is named sodium hydroxide.Name Chemical Compounds Step 6Bullet2.jpg
Naming Covalent Compounds
Understand a covalent compound. Covalent compounds are formed by two or more nonmetal elements. The name for the compound is based off how many atoms are present. The prefix attached is the Latin term for the number of molecules.
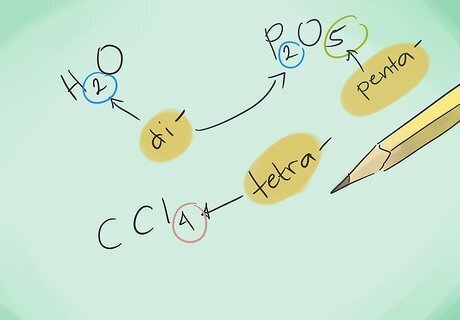
Learn the prefixes. Memorize the prefixes for up to 10 atoms: 1 atom – “Mono-“ 2 atoms – “Di-“ 3 atoms – “Tri-“ 4 atoms – “Tetra-“ 5 atoms – “Penta-“ 6 atoms – “Hexa-“ 7 atoms – “Hepta-“ 8 atoms – “Octa-“ 9 atoms – “Nona-“ 10 atoms – “Deca-“
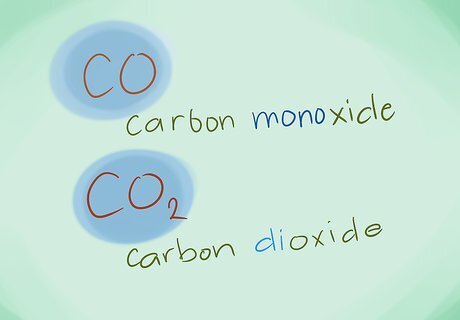
Name the compounds. Using the correct prefixes, name the new compound. You attach prefixes to any part of the compound that has multiple atoms. Example: CO would be carbon monoxide and CO2 would be carbon dioxide.Name Chemical Compounds Step 9Bullet1.jpg Example: N2S3 would be Dinitrogen trisulfide.Name Chemical Compounds Step 9Bullet2.jpg In most cases, the “mono-“ prefix can be omitted, because it is implied when it is not present. It is still used for carbon monoxide due to the term being in use since early chemistry.Name Chemical Compounds Step 9Bullet3.jpg

















Comments
0 comment