views
Using a Handheld Refractometer
Use this tool to accurately measure salinity in liquids. Refractometers measure how much light bends, or refracts, when it enters the liquid. This is called the refractive index. The more salts (and other material) dissolved in the water, the more resistance the light will meet and the more it will bend. This is because light travels at different velocities depending on the medium. The salinity of freshwater is less than 0.5 parts per thousand (ppt), while the salinity of saltwater is about 35 ppt. For a cheaper, but somewhat less accurate, method, try a hydrometer. If you are measuring the salinity of soil, use a conductivity meter.
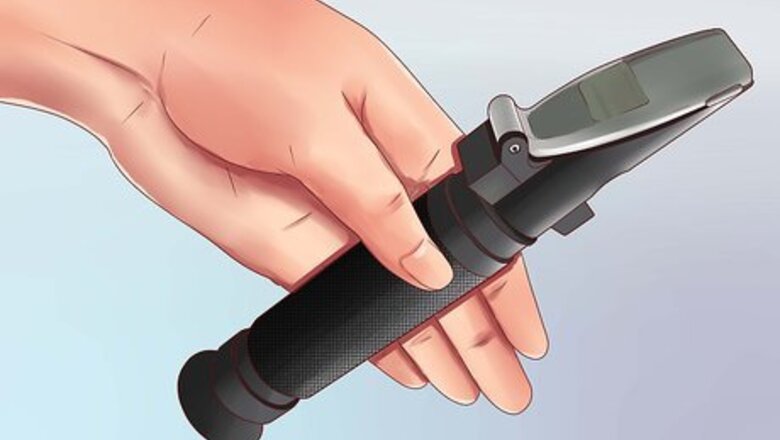
Select a refractometer designed for the liquid you wish to measure. Different liquids already refract light by different amounts, so to accurately measure additional salinity (or other solids content), use a refractometer designed specifically for the liquid you intend to measure. If the liquid is not specifically mentioned on the packaging, the refractometer is probably designed to measure salt water. Note: Salt refractometers are used to measure sodium chloride dissolved in water. Seawater refractometers are used to measure the mixture of salts typically found in seawater or saltwater aquariums. Using the wrong one may result in a reading approximately 5% off, which may be acceptable for non-laboratory purposes. Refractometers are also designed to compensate for the expansion of a specific material due to temperature changes.

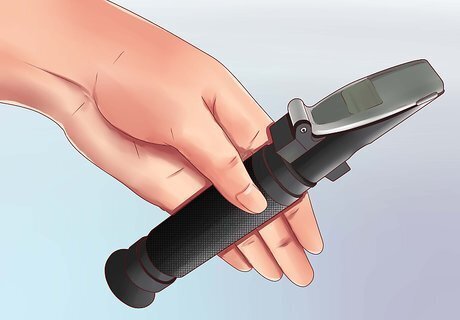
Open the plate near the angled end of the refractometer. A handheld refractometer has one round, open end to look through, and one angled end. Hold the refractometer so the angled surface is on the top of the device, and find the small plate near this end which can be moved to one side. Note: If you have not yet used your refractometer, you may wish to calibrate it first for a more accurate reading. This process is explained at the end of this section, but you may wish to read the following steps first so you are more familiar with how to work the device.

Add a couple drops of the liquid onto the exposed prism. Take the liquid you wish to measure, and use an eyedropper to pick up a couple drops of it. Transfer this to the translucent prism revealed when you moved the plate. Add enough water to completely cover the prism's surface with a thin layer.

Close the plate carefully. Cover the prism again by gently pushing the plate back into position. The parts on a refractometer may be small and delicate, so try not to apply much force even if they become slightly stuck. Instead, wiggle the plate back and forth with your finger until it moves smoothly again.
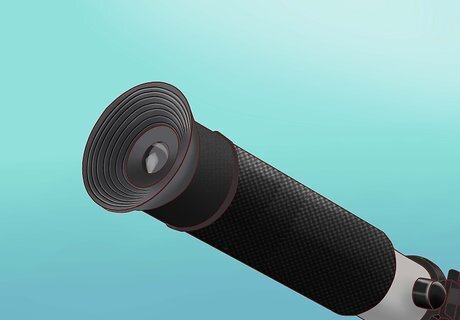
Look through the device to see the salinity reading. Look through the round end of the device. There should be 1 or more numbered scales visible. The salinity scale is likely labeled 0/00 meaning "parts per thousand", and ranges from 0 at the bottom of the scale, to at least 50 at the top. Look for the salinity measurement at the line where the white and blue areas meet.

Wipe the prism with a soft, damp cloth. Once you have the measurement you need, open the plate again and use a soft, slightly damp cloth to wipe the prism until it is free of water droplets. Leaving water in the prism, or immersing the refractometer in water, can damage the refractometer. A damp tissue may work if you don't have a cloth flexible enough to reach the whole surface of the tiny prism.

Calibrate the refractometer periodically. Periodically between uses, calibrate it to the correct reading by using pure, distilled water. Add the water to the prism as you would for any liquid, and check whether the salinity reading is "0." If not, use a small screwdriver to adjust the calibration screw, typically found beneath a small cap on the top or bottom of the device, until the salinity reading is "0." A new, high-quality refractometer may only need calibration once every few weeks or months. A cheaper, older refractometer may need to be calibrated before each use. Your refractometer may come with calibration instructions that specify a certain water temperature. If none are included, use room temperature distilled water.
Using a Hydrometer

Use this inexpensive tool for reasonably accurate water measurements. A hydrometer measures the specific gravity of water, or its density compared to pure H2O. This relies on Archimedes’ principle, which is that an upward force that is exerted on a body that is partly or fully submerged in fluid is equal to the weight of the fluid that is being displaced by the body. Because almost all types of salt are denser than water, a hydrometer reading can tell you how much salt is present. This is accurate enough for most purposes, such as measuring salinity in an aquarium, but many models of hydrometer are inaccurate or easy to use improperly. This method cannot be used for solid material. If you are measuring the salinity of soil, see the conductivity method instead. For a more accurate measurement, use the inexpensive evaporation method, or the faster refractometer method.

Narrow down your hydrometer options. Hydrometers, also called specific gravity meters, are sold online or at aquarium shops, in several different basic designs. Glass hydrometers that float in water are typically more accurate than other designs, but often don't have precise measurements listed (a longer decimal). Plastic "swing arm" hydrometers may be cheaper and sturdier, but tend to become less accurate over time.
Select a hydrometer with a listed temperature standard. Because different materials expand or shrink at different rates as they heat or cool, knowing the temperature the hydrometer was calibrated to is important when using it to calculate salinity. Select a hydrometer with a listed temperature on the device or packaging. It may be easiest to calculate salinity with hydrometers calibrated to 60 °F (16 °C) or 77 °F (25 °C) as these are the most common standards for measuring the salinity of salt water. You may use a hydrometer with a different calibration as long as it comes with a reference chart to convert its readings into salinity.
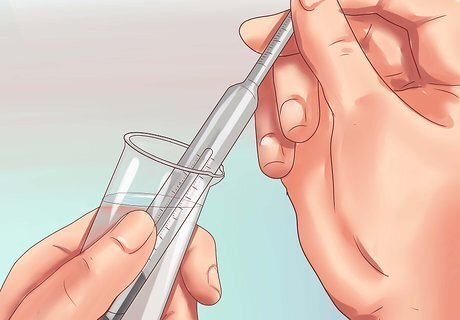
Take a sample of water. Transfer some of the water you plan to measure into a clean, transparent container. The container should be wide enough to fit the hydrometer, and the water should be deep enough to submerge most of the hydrometer. Make sure the container has been rinsed free of any dirt, soap, or other materials.
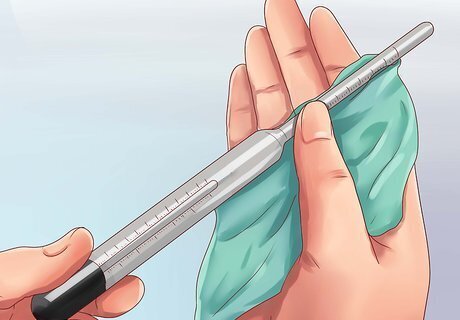
Measure the temperature of the water sample. Use a thermometer to measure the temperature of the water sample. As long as you know the temperature of your sample, and the temperature standard of your hydrometer, you can calculate the salinity. For a slightly more accurate reading, you may heat or cool the sample to the temperature the hydrometer was built for. Be careful not to overheat the water, as steaming or boiling can significantly alter the specific gravity.
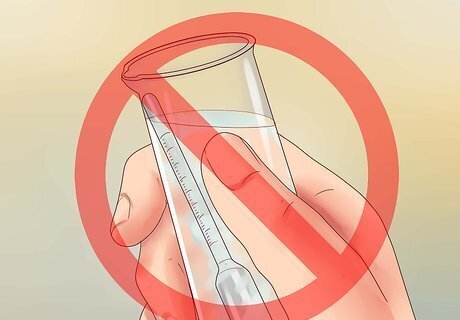
Clean the hydrometer if necessary. Scrub the hydrometer to remove any visible dirt or other solids on the surface. Rinse the hydrometer in fresh water if it was previously used in salt water, as salt may have built up on the surface.

Lower the hydrometer gently into the water sample. Glass hydrometers can be placed partially in the water, then released to float on its own. Swing-arm hydrometers will not float, and usually come with a small tab or handle that allows you to dip them into the water without getting your hands wet. Do not submerge glass hydrometers entirely, as this can mess up the reading.
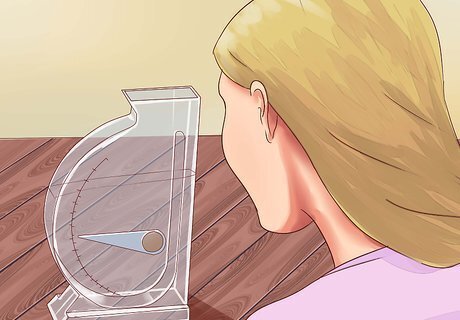
Shake gently to remove air bubbles. If air bubbles are clinging to the surface of the hydrometer, their buoyancy will result in the wrong density reading. Gently shake the hydrometer to remove these, then let the water turbulence settle down before continuing.

Read the measurement on a swing-arm hydrometer. Keep swing-arm hydrometers completely level, with no tilt in either direction. The measurement the arm points to is the specific gravity of your water.
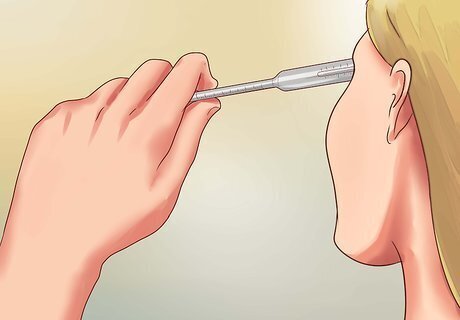
Read the measurement on a glass hydrometer. In a glass hydrometer, read the measurement where the surface of the water touches the hydrometer. If the water surface curves up or down to cling to the tool, ignore that curve and read the measurement at the level of the flat water surface. The water curve is called a meniscus, and is a phenomenon caused by surface tension, not salinity.

Convert the specific gravity measurement to a salinity measurement, if necessary. Many aquarium care instructions use specific gravity, typically measured between 0.998 and 1.031, so you do not need to convert to salinity, typically between 0 and 40 parts per thousand (ppt). However, if it only provides the latter measurement, you will need to convert between the first and second measurements yourself. If your hydrometer didn't come with a reference chart for this purpose, search online or in an aquarium reference book for a "specific gravity to salinity conversion" table or calculator. Make sure you use one designed specifically for the temperature standard listed on your hydrometer, or you may get the wrong result. This table is for hydrometers calibrated at 77 °F (25 °C) The water sample temperature is given in ºC. These charts and calculators will also vary based on the liquid, but the vast majority are used for salt water.
Using a Conductivity Meter

Use this to measure salinity of soil or water. An electrical conductivity meter, or EC meter, is the only common device that can be used to measure the salinity of soil. It can also be used to measure the salinity of water, but a high-quality EC meter may be significantly more expensive than a refractometer or hydrometer. Some aquarium hobbyists like to use both a conductivity meter and one of the other tools described on this page, in order to confirm their salinity reading.

Select an electrical conductivity meter. These devices send an electrical current through the material, and measure how much the material resists the flow of current. The more salts found in the water or soil, the higher the conductivity rating. In order to get an accurate reading for common water and soil types, select an EC meter that can measure up to at least 19.99 mS/cm (19.99 dS/m). S=Siemens, an electrical conductivity unit. mS=milliSiemens

Mix soil with distilled water, if you are measuring soil. Mix 1 part soil with 5 parts distilled water, shaking them together thoroughly. Let the mixture settle for at least 2 minutes before continuing. Because distilled water has no electrolytes or salts in it, the measurement you get will reflect the quantity of these materials in the soil. In laboratory conditions, you may be required to let the mixture settle for thirty minutes, or use a more accurate "saturated soil paste" method which can take over two hours. These are rarely done outside of a laboratory environment, however, and the method above is still reasonably accurate.
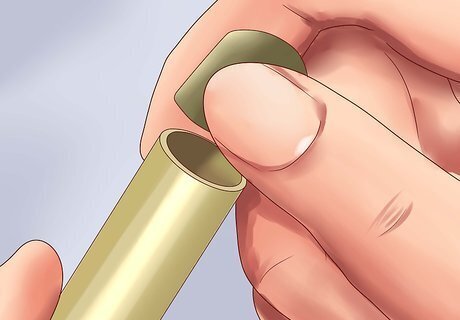
Remove the cap and dip the EC meter up to the required level. Remove the protective cap covering the thin end of the EC meter. Immerse this end to the level indicated on the meter, or just far enough that the thin probe is submerged, if no level is indicated. Most EC meters are not waterproof above a certain point, so do not drop the meter underwater.
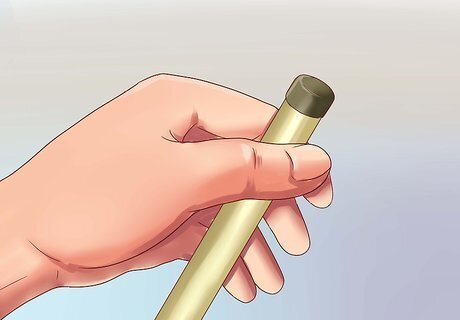
Move the meter gently up and down. This motion removes any air bubbles that are trapped inside the probe. Do not shake vigorously, as this could drive the water out of the probe instead.
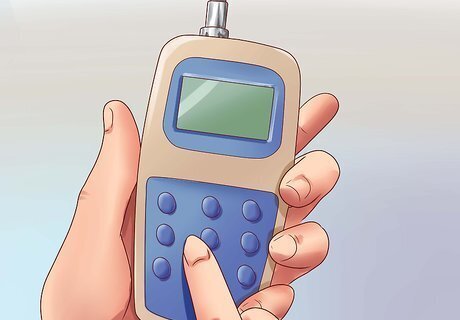
Adjust for temperature according to the EC meter's instructions. Some EC meters automatically correct for the temperature of the liquid, which can affect conductivity. Wait at least 30 seconds for the meter to make this adjustment, or longer if the water is unusually cold or hot. Other meters have a dial which can be manually adjusted to the correct temperature. If your EC meter has neither of these features, it may come with a chart which you can use to manually correct the reading based on the water's temperature.
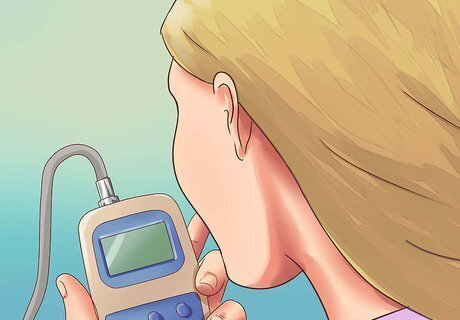
Read the display. The display is typically digital, and may give you a measurement in mS/cm, dS/m, or mmhos/cm. Fortunately, these 3 units are equal in size, so you do not need to convert between them. Respectively, these units stand for milliSiemens per centimeter, deciSiemens per meter, or millimho per centimeter. The mho (inverse of an ohm) is an old-fashioned name for the Siemens, but is still used in some industries.

Determine whether the soil salinity is appropriate for your plants. Using the method described here, EC readings of 4 or higher could indicate danger. Sensitive plants such as mango or banana may be affected at an EC as low as 2, while tolerant plants such as coconut may be fine with an EC as high as 8–10. Note: Whenever looking up EC ranges for a particular plant, find out which method that source used to test the EC. If the soil is diluted with 2 parts water, or with just enough water to make a paste, instead of the 1:5 ratio used here, the numbers may be significantly different.

Calibrate the EC meter periodically. Between each use, calibrate the EC meter by using it to measure an "electrical conductivity calibration solution," purchased for this purpose. If the measurement does not match the known conductivity of that solution, use a small screwdriver to adjust the calibration screw until the measurement is correct. Some calibration solutions may come with a "check solution" to test after calibration. If the check solution's conductivity is inaccurate, your EC meter may be broken.



















Comments
0 comment