
views
Preparing the Salt Bridge

Dissolve the potassium nitrate in water to form a solution.
Cut out a strip of filter paper.

Soak the filter paper with the solution.
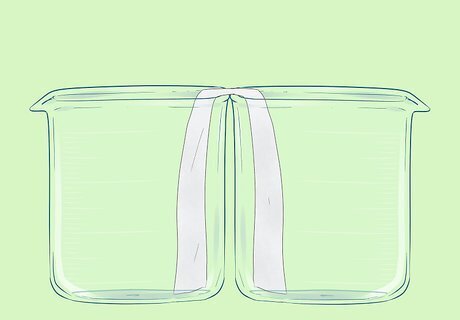
Place the two beakers next to each other. Bend the filter paper so that it touches the bottom of both beakers.
Preparing the Negative Half-Cell
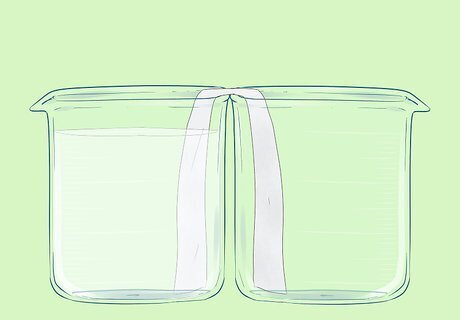
Add the aluminium nitrate solution to one of the beakers.
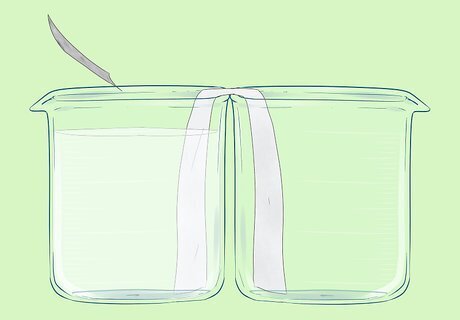
Place a strip of aluminium foil into the solution. The strip should touch the bottom of the beaker. It should not touch the salt bridge. You may wish to bend the top of the strip over the edge of the beaker. This aluminium strip is the electrode. You have formed an Al3+/Al half cell.
Preparing the Positive Half Cell
Gather your supplies. You will need: a strip of copper and some one molar copper nitrate solution.
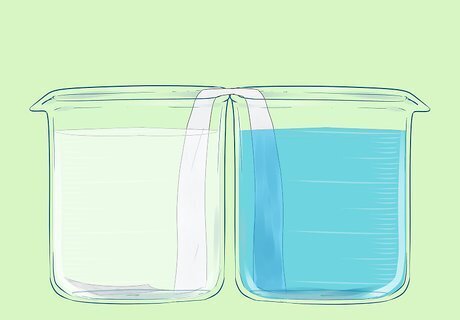
Add the copper nitrate solution to the second beaker.
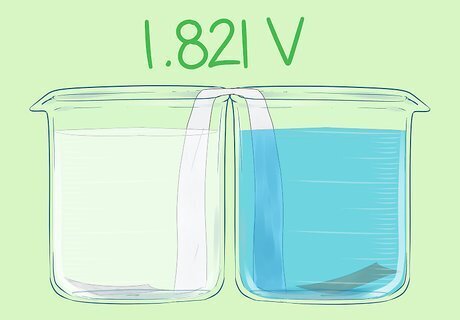
Place the copper strip in the second beaker. It should touch the bottom, but not the salt bridge. This is the positive electrode. This cell has a voltage of 1.821V.

Gather your supplies. You will need: one molar iron (III) nitrate (Fe(NO3)3) solution, one molar iron (II) nitrate (Fe(NO3)2) solution, and a conductive graphite rod.
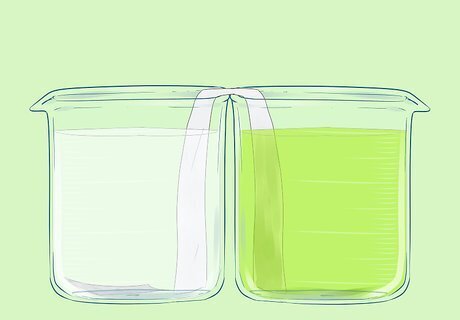
Add equal amounts of each of the iron nitrates to the second beaker.
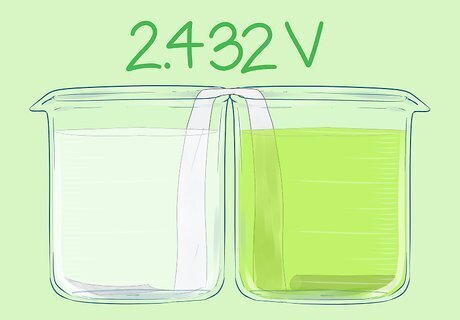
Place the graphite rod into the beaker. It should not touch the salt bridge. This is the positive electrode. This cell has a voltage of 2.432V.
Get the supplies for this part. You will need: one molar potassium dichromate solution, one molar nitric acid, one molar chromium nitrate solution, and a conductive graphite rod.
To the second beaker, add: one measure of potassium dichromate solution, two measures of chromium nitrate solution, and an excess (>14 measures) of nitric acid. One measure should be the largest amount that you can add without the beaker overflowing after everything is added.
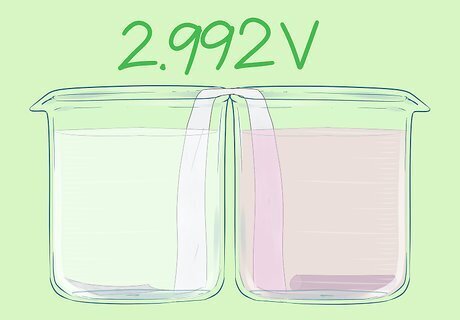
Add the graphite rod. It should not touch the salt bridge. This is the positive electrode. This cell has a voltage of 2.992V.
Gather the correct supplies. You will need: one molar potassium permanganate solution, one molar nitric acid, one molar manganese nitrate solution, and a conductive graphite rod.
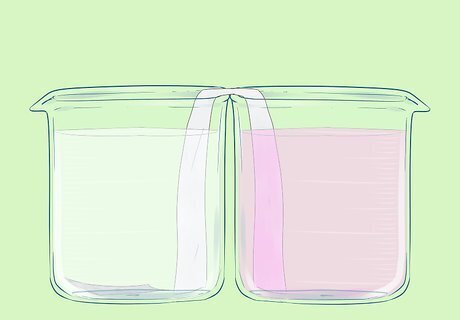
To the second beaker, add: one measure of potassium permanganate solution, one measure of manganese nitrate, and an excess (>8 measures) of nitric acid. One measure should be the largest amount that you can add without the beaker overflowing after everything is added.
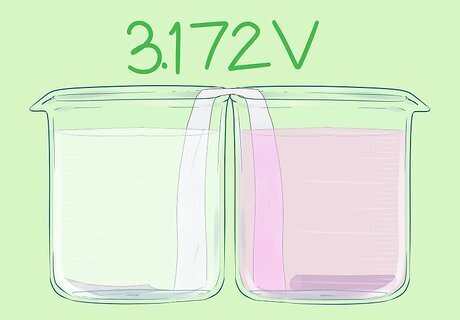
Add the graphite rod. It should not touch the salt bridge. This is the positive electrode. This cell has a voltage of 3.172V.
Completing the Circuit
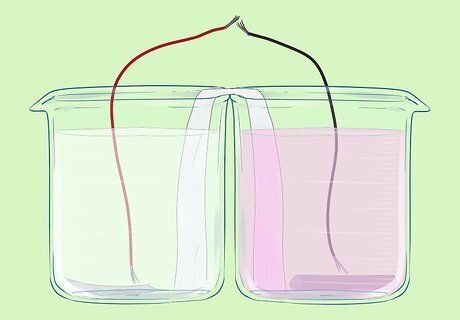
Connect wires to each of the electrodes.
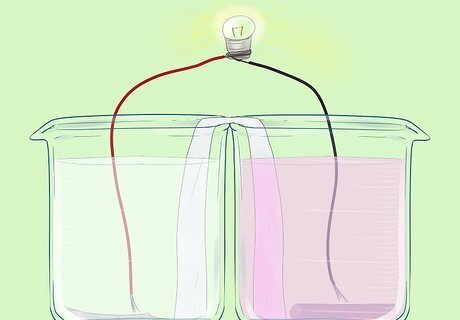
Connect these wires to the circuit (a light bulb). The bulb should light up.



















Comments
0 comment