views
Basic Concepts
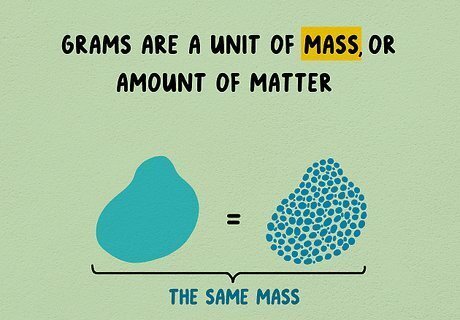
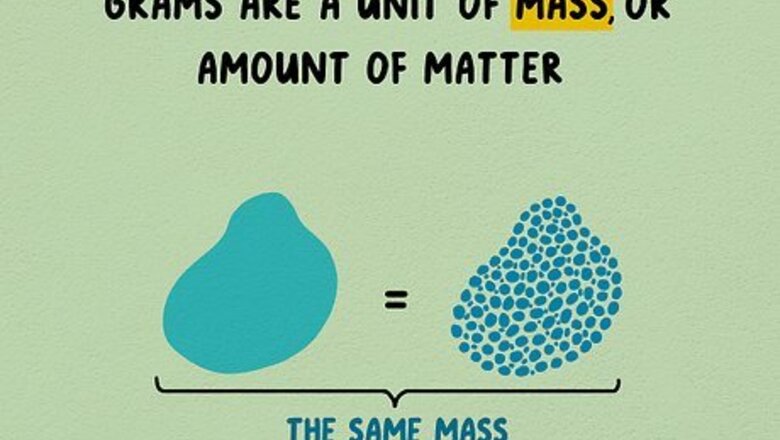
Understand grams and mass. Grams are a unit of mass, or amount of matter. If you crush an object to make it smaller and denser, this will not change its mass. A paper clip, sugar packet, or raisin all weigh about one gram. The gram is often used as a unit of weight, and it can be measured using a scale in everyday situations. Weight is a measurement of the force of gravity on mass. If you traveled to space, you would still have the same mass (amount of matter), but you would no longer have any weight, since there would be no gravity. The gram is abbreviated g.
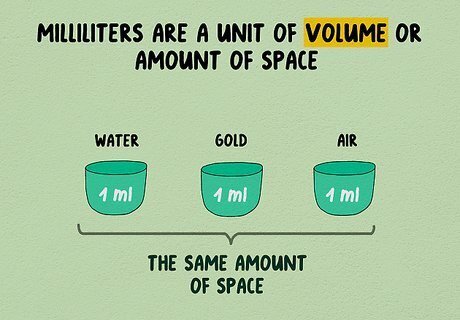
Understand milliliters and volume. Milliliters are a unit of volume, or amount of space. One milliliter of water, one milliliter of gold, or one milliliter of air will all take up the same amount of space. If you crush an object to make it smaller and denser, this will change its volume. About twenty drops of water, or 1/5 of a teaspoon, have a volume of one milliliter. The milliliter is abbreviated mL.
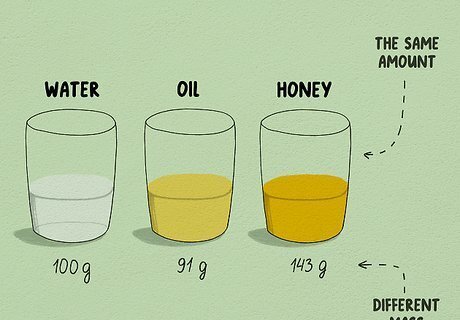
Learn why you need to know which substance you are converting. Since these units measure different things, there is no quick formula to convert between them. You have to find the formula based on the object you are measuring. For example, the amount of molasses that fits into a one milliliter container will weigh a different amount than the amount of water that fits into a one milliliter container.
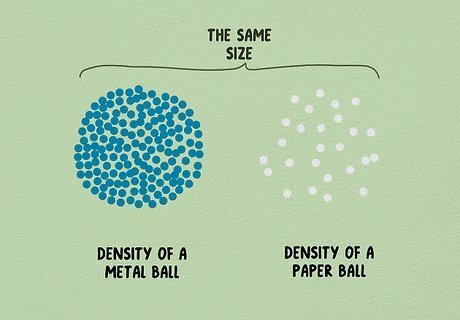
Learn about density. Density measures how closely the matter in an object is packed together. We can understand density in everyday life even without measuring it. If you pick up a metal ball and are surprised at how heavy it is for its size, that's because it has high density, packing a lot of matter into a small space. If you pick up a crumpled ball of paper of the same size, you can throw it around easily. The paper ball has low density. Density is measured in mass per unit volume. For instance, how much mass in grams fits into one milliliter volume. This is why it can be used to convert between the two measurements.
In the Kitchen
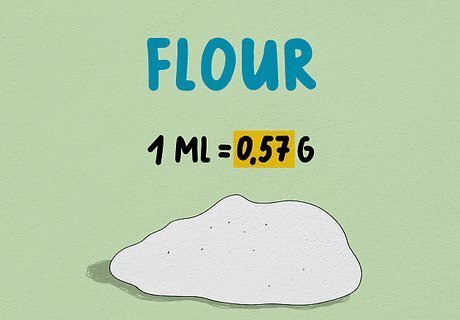
To convert for flour, multiply by 0.57. There are many types of flour, but most brands of all-purpose, whole wheat, or bread flour fall pretty close to the same density. Because of the possibility of variation, however, add the flour to your recipe bit by bit, using less or more if necessary based on how the dough or mixture appears.This measurement was calculated based on a density of 8.5 grams per tablespoon, and a conversion of 1 tbsp = 14.7868 mL.

To convert for milk, multiply by 1.03. Multiple the mL measurement for milk by 1.03 to get its mass (or weight) in grams. This measurement is for whole, full-fat milk. Skim milk is closer to 1.035, but this difference is not significant for most recipes.

To convert for butter, multiply by 0.911. If you do not have a calculator, multiplying by 0.9 should be easily accurate enough for most recipes.

To convert measurements of water, do nothing. One milliliter of water has one gram of mass, and weighs one gram in typical situations, including for cooking recipes and math and science problems (unless another stated). There is no need to do any math: the measurement in milliliters and grams are always the same. This easy conversion is not a coincidence, but a result of how these units were defined. Many scientific units are defined using water, since it is such a common and useful substance. You only need to use a different conversion if the water is much hotter or colder than possible in everyday life.
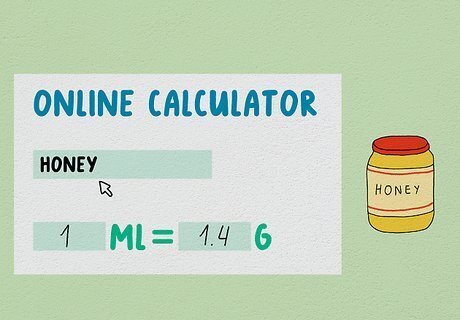
Use an online calculator for other ingredients. Most common foods can be converted using the aqua-calc food converter online. A milliliter is the same as a cubic centimeter, so select the "cubic centimeter" option, enter the volume in milliliters, then type in the food or ingredient you wish to convert.
Converting Any Substance
Look up the substance's density. As described above, density is mass per unit volume. If you are answering a math or chemistry problem, it may tell you what the substance's density is. Otherwise, search for the substance's density online or on a chart. Use this chart to look up the density of any pure element. (Note that 1 cm = 1 milliliter.) Use this document to look up the density of many foods and beverages. For items that only have a "specific gravity" listed, that number equals the density in g/mL at 4ºC (39ºF), and will typically be reasonably close for substances at roughly room temperature. For other substances, type the name of the substance and "density" into a search engine.
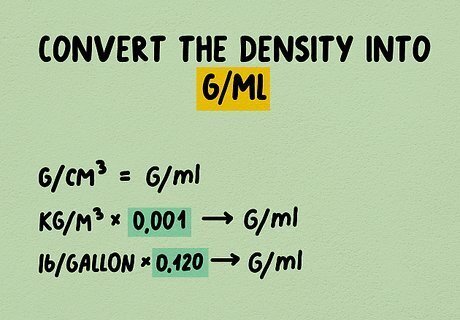
Convert the density into g/mL if necessary. Sometimes, the density is given in units other than g/mL. If the density is written in g/cm, no changes are necessary, since a cm is exactly equal to 1 mL. For other units, try an online density conversion calculator, or do the math yourself: Multiply the density in kg/m (kilograms per cubic meter) by 0.001 to get the density in g/mL. Multiply the density in lb/gallon (pounds per U.S. gallon) by 0.120 to get the density in g/mL.
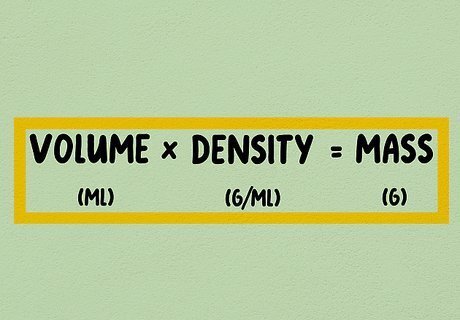
Multiply the volume in milliliters by the density. Multiply the mL measurement of your substance by its density in g/mL. This gives you an answer in (g x mL) / mL, but you can cancel the mL units at the top and bottom and end up with just g, or grams. For example, to convert 10 mL of ethanol to grams, look up the density of ethanol: 0.789 g/mL. Multiply 10 mL by 0.789 g/ml, and get 7.89 grams. Now you know that 10 milliliters of ethanol weighs 7.89 grams.



















Comments
0 comment