views
With more than 6.5 million people known to be infected, and about 400 thousand recorded deaths, Covid-19 shows no signs of relenting, although the devastation is gradually shifting from the initial hotspots to countries like Brazil, Mexico, Russia and India.
The global race to find a vaccine or a cure is continuing with no clear breakthrough yet. Reportedly, nearly 100 vaccines are in development, and about 10 of them are already in clinical trial phase. Global pharmaceutical giants are locked in a race with each other and against time, to repurpose existing drugs as treatment options for Covid-19 remain extremely limited, and the frantic search continues.
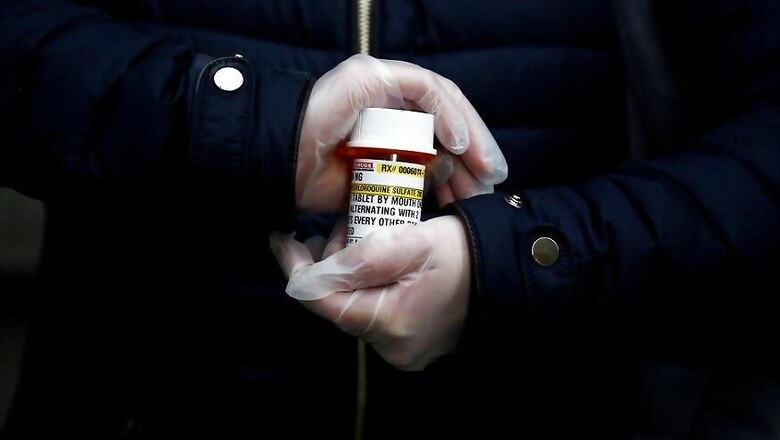
If Covid-19 had a higher infection fatality rate, much of current debate around the use of Hydroxychloroquine (HCQ) in prophylaxis and treatment would have become irrelevant. Known to be far safer than Chloroquine, HCQ still has serious side-effects for people with certain health conditions.
Since many who take it to deal with Covid-19 are oblivious to their own health conditions, it makes them vulnerable to side-effects. HCQ’s use as a mass prophylaxis and treatment with uncertain (if any) benefits for a disease that is relatively mild for the majority of population, is therefore being widely contested.
Many experts raised concerns in the past with regards to ICMR recommending HCQ as prophylaxis for asymptomatic health workers caring for suspected or confirmed Covid-19 cases and for asymptomatic household contacts of confirmed patients, given lack of compelling evidence for its effectiveness.
Despite this, many states in India have been using HCQ as prophylaxis as well as for treating Covid-19, because of lack of affordable and effective alternatives. To this end, ICMR has published a study which found no major side effects of HCQ and recommended that it should be continued as prophylaxis.
On 22 May, the Government of India expanded the use of HCQ as prophylaxis to front-line workers, including the police and healthcare staff conducting door-to-door surveys to estimate Covid19 burden. Apart from the gravity of the situation, ICMR’s decisions were perhaps also driven by the need to prevent panic on the frontlines and as a stopgap solution. This step was likely to be seen as better than no solution at all.
However, after a major Lancet study published in May suggested no evidence of benefit from HCQ and instead pointed to an increase in the risk of abnormal heartbeats and a greater hazard for in-hospital death in Covid-19 patients, there was fresh public outcry against ICMR’s decision.
Another double-blind, randomized trial study in the prestigious New England Journal of Medicine (NEJM) published on 3rd June found that HCQ administered to people exposed to the virus did not show any benefit. However, the study stated that it remains theoretically possible that HCQ limits proven infection, and called for further examination of evidence.
Interestingly, the study did not explore the effectiveness of pre-exposure prophylaxis which is at the centre of controversy in India, but found that among the 414 participants that received HCQ, no serious adverse reactions were reported.
Over the past few days, publications including the Lancet and the NEJM raised red flags about possibility of data manipulation by the group who published the Lancet study, and both articles under the cloud of suspicion were retracted by the lead author along with majority of authors who complained they “no longer vouch for the veracity of the primary data sources”, indicating there was misconduct by the controversial analytics company in question called Surgisphere Corporation.
For their part, Surgisphere and its CEO Sapan Desai maintain that data is not shared with co-authors because of client agreements and confidentiality requirements of the company.
Ever since HCQ was endorsed by President Trump as an effective weapon to fight coronavirus, a worldwide surge in demand was witnessed. As the largest global producer, this also became a potent tool for India’s health diplomacy efforts. This intermingling of global politics, scientific disagreements and ever sharpening commercial agendas (due to the investments into drug and vaccine development) make the debate on HCQ a vexed one.
An article published in the Indian Journal of Public Health on June 2 reviewing evidence on the use of HCQ as prophylaxis as well as treatment found that currently there is no compelling evidence for, or against HCQ’s use against Covid-19.
The review also recommended that it is unsafe to use HCQ in combination with azithromycin. Given all the studies and pronouncements that have been put out, the key rationale for its use on the frontlines may be dictated by the affordability of the drug, lack of options and not having the luxury of time to wait for conclusive answers.
Following the controversial Lancet study, the quick decision by WHO and affiliated research institutes around the world to pause the HCQ arm of the Solidarity Trial is currently being criticised widely. The decision has been since then reversed.
In the meantime, indiscriminate use of HCQ citing the tentative ‘stamps of approval’ by ICMR, WHO and President Trump, without screening out people with risky conditions or constant monitoring for adverse reactions remains a worry. This is particularly true for India where surveillance systems remain weak. The Government of India seems to have chosen to play down the risk, and stick with the HCQ option.
It is also likely that HCQ is being used to cover up for the failure of governments to provide healthcare workers with personal protective equipment (PPE), even in the most advanced economies. For example, about 60% of the participants of the NEJM study-mostly healthcare workers from the USA and Canada- reported not wearing any element of personal protective equipment during their Covid-19 exposure.
Shortages of PPE across Indian hospitals is well documented, and a false sense of security that HCQ provides may do more harm than good. With the reported and dramatic increase in domestic PPE production this fear may also be addressed, thereby making the debate on the use of HCQ less relevant.
The fog of war surrounding Covid-19 and the desperation of the scientific community and practitioners to have evidence-informed medicines and vaccines may give room for large- scale manipulation of studies, like the Lancet controversy proves.
Interestingly, the “declaration of interests” statement accompanying the retraction notice in Lancet by the authors had more information than the one accompanying the original article itself. It revealed the commercial links of the authors with key global pharmaceutical companies and will likely fuel the controversy even further.
As Covid-19 is a self-limiting disease for a majority of patients, true effectiveness of any drug should be identified only through rigorous scientific enquiry. Any commercial interest meddling with that process by mediating in knowledge production needs to be resisted and punished, as the scope for profiteering by unethical corporate interests is high, given the real and perceptional threat and panic that proliferate within most communities.
The article first appeared in ORF.


















Comments
0 comment