views
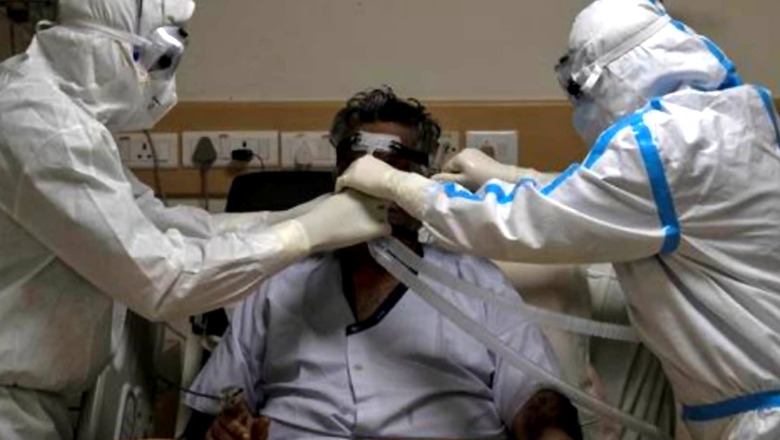
It may be premature to conclude that complications like lung fibrosis and increased thrombotic events due to COVID-19 are on the rise. However, a larger number of cases of fungal infections have been found linked with the coronavirus infection during the recent surge, the government informed Parliament on Friday. In a written reply to a question in the Lok Sabha, Minister of State for Health Bharati Pravin Pawar said the Indian Medical Association (IMA), in a letter dated June 7 addressed to the prime minister, mentioned that post-Covid complications such as lung fibrosis, thrombotic events and fungal infections are on the rise and there is a need to prepare for the same.
It also urged the government to set up a separate research cell to study post-Covid complications and come out with multi-faceted treatment guidelines in all disciplines of medicine, following which expert group consultations under the director general of health services are going on to review the emerging evidence on organ system specific (respiratory system, renal system, cardiovascular and gastro-intestinal) sequelae of COVID-19. Pawar further said the ICMR has established a National Clinical Registry on Covid with an objective to collect data regarding clinical and laboratory features, treatments and outcomes of hospitalised Covid patients in the country and to study the frequency, clinical and laboratory features, treatments and outcomes of Covid-related multi-system inflammatory disorder in children and adolescents by analysing the registry. The objective is to utilise the data to answer research questions on COVID-19, including the natural course of the disease, the spectrum of the disease, prognostic factors, outcome data, medications, health systems and context-specific questions such as COVID-19 in tuberculosis, malnutrition etc.
The National Clinical Registry on COVID-19 has been established to serve as a platform for additional clinical research studies in selected sites and to collect follow-up data of discharged Covid patients, if possible. The registry follows a hub-and-spoke model with primary data being collected in the electronic data capture form by satellite centres (dedicated COVID-19 hospitals), which are being trained, mentored and supervised by medical institutes of national repute chosen region-wise.
In the first phase, 50 satellite centres and 14 registry sites have been included. In addition, all AIIMS-like institutions have been requested to undertake research to study the long-term impact of COVID-19, Pawar said. "It may be premature to conclude that complications like lung fibrosis and increased thrombotic events directly due to COVID-19 infection are on rise. However, a larger number of cases of fungal infections have been noted linked with COVID-19 during the recent surge," she added.
Responding to a question on whether the drugs needed for the mucormycosis fungal disease are not available with ease, Pawar said although mucormycosis is not a new disease, its true incidence was unknown as it was not a notifiable disease. The health ministry urged the states in May to declare mucormycosis a notifiable disease under the Epidemic Diseases Act, 1897 to get an objective assessment of the disease in the community.
Since early May, the details of production, stock, supplies made and purchase orders were obtained from the manufacturers and their co-operation was sought to overcome the gap between supply and demand. Both first and second-line drugs used for managing mucormycosis cases — Amphotericin B Deoxycholate and Posaconazole — are amply available in the Indian markets. The government has taken various measures to improve the availability of Amphotericin B (Liposomal) through a multi-pronged approach of augmenting production and import and ensure equitable distribution among the states. Elaborating on the measures initiated, Pawar said the Centre is continuously engaging with the manufacturers to resolve their issues related to raw materials, and the Department of Pharmaceuticals and the Drug Controller General of India (DCGI) have actively coordinated with the industry for the identification of manufacturers, alternate drugs and expeditious approvals of new manufacturing facilities.
The five existing manufacturers have also been called upon to increase the production of Liposomal Amphotericin-B. The DCGI, after consultations with the association of drugs manufacturers, has issued manufacturing or marketing permission for the Amphotericin B Liposomal injection to six additional firms. Various concerns of the manufacturers and importers, including those related to licensing and availability of raw material and import licence, are being speedily addressed, Pawar said.
The Ministry of External Affairs (MEA) is reaching out to various manufacturers abroad to identify new sources of Amphotericin B or Liposomal Amphotericin B injections and alternative drugs for the treatment of mucormycosis. It has also been actively working on ensuring supplies of key excipients from sources abroad for the production of Liposomal Amphotericin B in India. For equitable distribution, allotments are being made to the states and Union territories in accordance with the proportion of their reported caseload in respect of the entire country and the supply arrangements are being monitored by the National Pharmaceuticals Pricing Authority (NPPA) to ensure expeditious availability of the drug, the reply stated.
Read all the Latest News, Breaking News and Coronavirus News here.



















Comments
0 comment