views
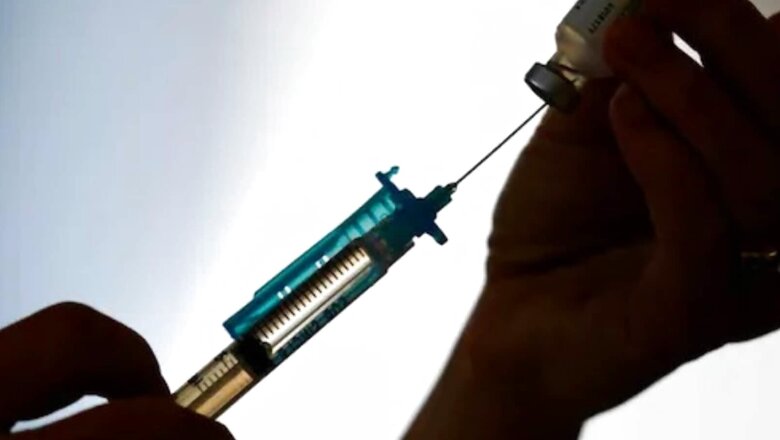
The Phase2/3 trials of India’s first indigenous mRNA vaccine on humans has been completed, a report has suggested. Pune-based Gennova Biopharmaceuticals is now in the process of submitting the data to the country’s drug regulator.
According to a report by The Indian Express, plans are also on to conduct the Covid-19 vaccine’s Phase 2 trials on children. “Phase II and III trials have been completed. We have submitted and presented Phase II data and are in the process of submission of Phase III trial data that was conducted among 4,000 participants,” the publication quoted Dr Sanjay Singh, CEO of Gennova Biopharmaceuticals, as saying.
Last week, responding to a question at the weekly press conference, NITI Aayog Member (Health) Dr V K Paul said a candidate vaccine by Pune-based Gennova Biopharmaceuticals, which is entirely an Indian development, is in final clinical trial stages.
The Drug Controller General of India had in August last year, gave its nod to conduct Phase 2 and Phase 3 study protocols for HGCO19, which is India’s first homegrown mRNA-based Covid-19 vaccine developed by Gennova Biopharmaceuticals Limited (“Gennova”).
Gennova had thereafter submitted the interim clinical data to the Central Drugs Standard Control Organisation for its Phase 1 trials.
In last week’s press conference, VK Paul said, “We hope that it will pass that threshold that it could be used under emergency use and regular use some day.” The official noted that the vaccine can be stored in normal cold chain conditions and transported, which is a big thing.
“o we have a great candidate. They have also tweaked it for Omicron variant that will come forward. We need the mRNA platform because it is a new platform and it has been shown that vaccines developed on these platforms at least for coronavirus have been effective worldwide,” Paul added.
The trial was conducted at DBT-ICMR clinical trial network sites, The Indian Express said in its report. Sources of the publication at the Department of Biotechnology (DBT), Government of India, have also said that the trial data will have to be studied and checked following which a rollout may be planned.
For clinical trials on kids, the challenge will be to enroll participants amid a large number of Covid-19 infections, where the paediatric population is likely to be asymptomatic. In this scenario, even as scientists seek answers, certain criteria need to be decided upon. Participants, however, would require a Covid-19 negative report.
“The trial design protocol for a vaccine trial in the paediatric age group is still under discussion . Once the regulatory approvals are obtained, the trial will commence in a fortnight’s time,” Singh told The Indian Express.
ICMR Director General Balram Bhargava said this is another vaccine which really establishes that India is heading towards becoming a vaccine super-power and the fact that these vaccines are going to be available for other diseases. and because such a large proportion has been vaccinated, we are not seeing such a disastrous third surge in terms of hospitalisation and mortality, he said.
(With PTI inputs)
Read all the Latest India News here















Comments
0 comment