views
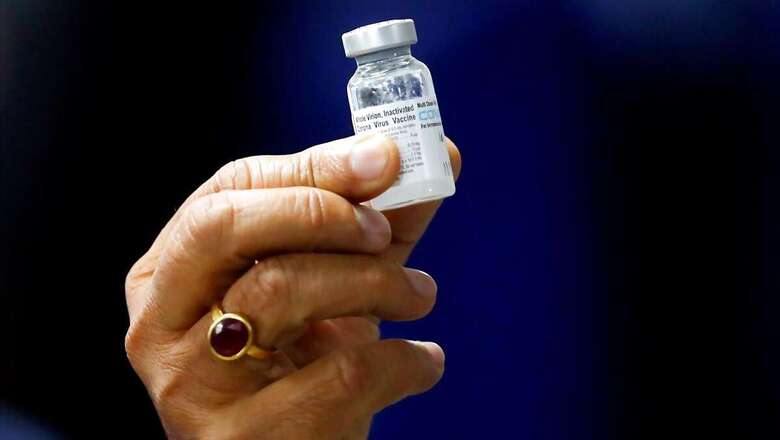
The Subject Expert Committee (SEC), a group of independent experts that advises the national drug regulator, is likely to meet to review ‘made in India’ Covaxin’s phase three trial data on Tuesday. The meeting is being held after Bharat Biotech submitted the data to the Drug Controller General of India (DCGI) over the weekend.
The phase 3 trial data will clearly state the efficacy of the Covid-19 vaccine that has been developed by the Hyderabad-based firm in association with the Indian Council of Medical Research. The developers had already been in the dock for delay in announcing its phase 3 trial data.
The DCGI in January had granted permission for emergency use of Covaxin based on its phase 1 and 2 clinical trials that comprised about 680 participants.
Bharat Biotech is set to hold its pre-submission meeting with the World Health Organization (WHO) on Wednesday (June 23) for the listing of Covaxin. The approval by WHO will make it easier for made-in-India vaccine recipients to travel abroad.
In a bid to get life back to normal, some nations have been mulling to roll out a so-called vaccine passport — an easily accessible and verifiable certification that a person’s been inoculated. Private companies have already begun to look at making Covid shots compulsory for people who want to travel on planes, cruise ships or attend public events such as concerts.
The pharmaceutical company has been facing hurdles in getting made-in-India vaccine approved in some foreign countries since it lacks Phase-3 trial data, which is essential for a WHO nod to export the vaccine and make it a part of the much-coveted coronavirus “vaccine passport”.
Bharat Biotech had earlier said that it will make phase three trial data public during July, following which the company will be applying for full licensure of the Covid-19 vaccine in India.
The company had earlier said that they would do Phase-4 trials to check the “real-world effectiveness of the vaccines” and to meet scientifically approved standards for safety and efficacy.
Read all the Latest News, Breaking News and Coronavirus News here.


















Comments
0 comment