views
Understanding the Periodic Table
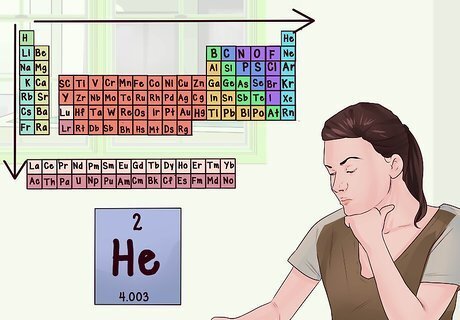
Know the direction of increasing atomic mass. Atomic mass (the amount of matter in your atom) increases as you go to the right across a row, or down a column. The periodic table is ordered in such a way that each element has one proton more than the element before it, and the mass is the sum of protons and neutrons in an atom. Electrons are negligible when calculating mass because they possess so little in comparison to the larger particles.
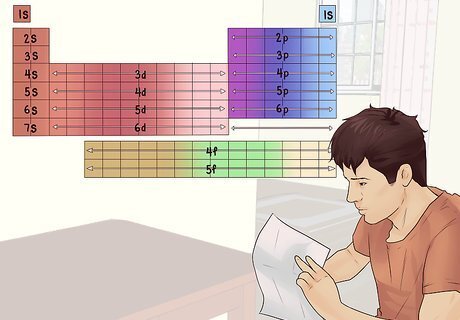
Think about outer shell electrons. The inner shells of an atom’s electrons are full and stable. For the most part, they contribute little to the way the atom reacts. The largest factor in how an atom reacts is the makeup of its outer shell electrons. The periodic table naturally groups together elements with similar outer shells (Transition metals, halogens, etc.).
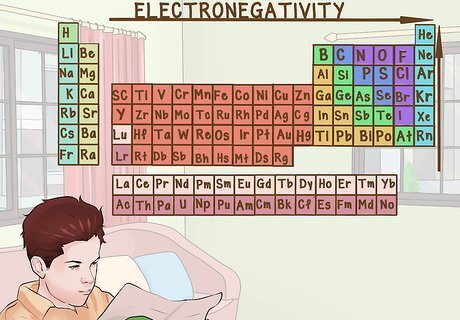
Consider how tightly an atom holds its electrons. For a number of reasons, an electron closer to the nucleus of an atom will generally be held tighter than an electron further out. Because of this, electronegativity (ability to accept and keep electrons) increases as you go to the right of a row and the top of a column. The noble gases (the column furthest to the right) are typically inert and do not accept or donate electrons. For example, fluorine is will more effectively gain electrons than bromine, even though they are both halogens.
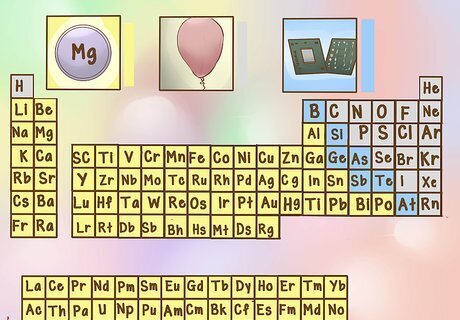
Understand metallic and nonmetallic properties. The periodic table also groups elements into metals, non-metals, and metallic categories. Metals are usually shiny solids at room temperature, conduct well, and are malleable. Non-metals are usually dull in appearance, may be solid, gas, or liquid at room temperature, conduct poorly, and are not malleable. Metalloids share properties of metals and non-metals. There are also different groups of metals (alkali metals, transition metals, etc.).
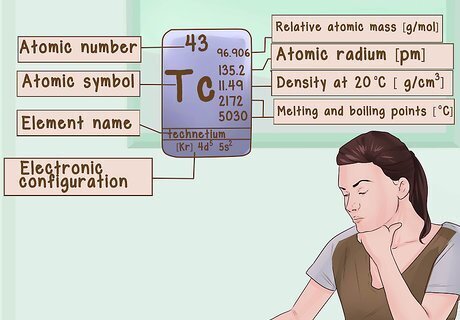
Read the information in each block. Each block of the periodic table contains the atomic symbol if one of the elements. There is also a lot of information about the element in that block including things like mass number and average atomic weight. Learn to Read the Periodic Table well, and you will be on your way to understanding the elements it contains.
Memorizing the Periodic Table
Use visualization techniques to remember elements. Your brain is designed to remember visuals more effectively than written or spoken words. Use this to your advantage by creating mental visualizations to go with each element. Then, you can create a storyline that likes each picture together in the order that you need to remember them. This is the most effective memorization technique. For example, you might drop your bottle of hydrogen peroxide (hydrogen), causing you to yell in a squeaky voice (helium makes your voice squeaky), right as it lands on a pack of batteries (lithium ion batteries to be exact). This helps you remember the order of elements within the periodic table.

Make up acronyms or acrostics to remember chunks of the periodic table. This is less effective than visual imagery, but can work better than brute force repetition. While acrostics are much easier to create for the elements, acronyms can be done, too. This will provide a cue for several elements in one word or phrase. An acrostic for nitrogen, oxygen, fluorine and neon might be something like No Openings For Ned. The first one or two letters of each word represent an element (N, O, F, Ne). HONClBrIF (pronounced honkle brief) is another acronym. This one helps you remember the elements that occur as diatomic molecules. A similar approach to acronyms and acrostics is to make a song that includes the names of the elements. This can be a little more effective, but is also more time consuming.

Employ brute force repetition. This method is least effective overall, and requires an intense amount of study time. It is best if the study time is broken up into smaller sessions. You will need to memorize small chunks of the table at one time and constantly review them while moving on to other chunks. If you choose this way, notecards are your best friend. For example, you could make a set of notecards for every element on the periodic table. Likewise, you could make a set of notecards that lists every family or group (column) and include the elements within each group on the card. To get the most out of the table, you could make several sets of notecards, such as the two mentioned, that organize the information in different ways.
Utilizing Periodic Table Games and Activities
Take practice tests. Quiz yourself relentlessly on the periodic table. Practice tests are among the most effective study habits because they force your brain to recall the information that you have committed to memory. You can search for practice tests online, or print off versions of the periodic table that need some parts filled in. You can also write your own practice test questions or make your own tables. This takes more time, but you will be thinking even more about the periodic table by formulating the test. Just be sure to ask questions that you don’t know, too!

Reward yourself for knowing your notecards. You can play fun games with yourself by rewarding yourself each time you get a notecard correct. It does not have to be a big reward to be fun. Even just a piece of candy for each correct answer can break up the boredom of studying. You can also give yourself bigger rewards for getting bigger chunks of information correct.

Use online games. There are several educational games online. Go to Google and type in “periodic table games and activities” and you will find an array of free games that help you learn the periodic table. Choose one you like and start playing.
Improving Your Study Habits

Make and keep a study schedule. A solid study schedule is a good way to make sure that you are spreading out your study time. It will help you remain disciplined in your studies and keep you motivated on days that you are less than excited to look at the periodic table again. While there’s no set rule on how much time it will take you to learn about the elements on the table, you should expect to set aside about two hours of study time for every hour of class time dedicated to the periodic table.

Know what kind of learning style you have. There are many different ways to expose yourself to new material, and most people prefer one or two over the others. In the classroom, the instructor often dictates how material will be presented. However, during your study time, you should strive to present yourself with material in the way that you learn it best. The different learning types are: Visual - You prefer to include pictures, images, videos, etc. Auditory - You like hearing information and/or integrating music and sounds. Linguistic - You learn information when it is in the form of language (written or spoken). Kinesthetic - You are a “hands on” learner. Movement and physical touch help you retain information. Mathematical - You rely on systems of logic to learn. Interpersonal - Most of your learning occurs in a group setting. Intrapersonal - Learning alone suits you best.

Spread out your studying. Research shows that cramming does not work. You will retain much more information with the same amount of study time if you spread it out over the course of days or weeks. Divide the periodic table into easy to conquer goals and learn it slowly over time. You should also take a study break every forty-five minutes or so to let your brain rest. Be sure that you go back and review material that you have already learned from time to time. If you keep moving on to new material without reviewing the old, you are likely to forget some important details. This can be a great way to use your notecards. Keep them handy, and when you find yourself with a few minutes to kill in a waiting room or between classes, pull them out and put that time to use.

Spend time studying in a group. Even if you prefer to study alone, you can benefit from a little bit of group study. This is where you will see different points of view on the same material and expand your understanding of the periodic table. Other students may notice trends that you weren’t familiar with, or they might have unique ways of learning the elements. Be sure that you contribute your ideas and knowledge to the group as well. Another way that you can utilize social learning is to teach someone else about the periodic table. As you teach them, you will become more familiar and comfortable with the table yourself. If you are struggling with learning the periodic table, you can also consider finding a private tutor. Sometimes the class will move on and you will simply be given the assignment to learn certain parts of the periodic table.

Get enough sleep every night. The periodic table is full of vast amounts of information on chemical elements, groups, and recurring trends that are found in similar elements. This can be a lot of information to commit to memory, and information is best organized and retained in your brain during sleep. Staying rested by getting seven to nine hours of sleep per night will do wonders for your study results.

Use unique scents to activate your memory. Your brain’s memory system is closely tied to your olfactory sense (sense of smell). You can use this to your advantage by studying in the presence of a unique (ideally inoffensive) smell. If you then take an exam in the presence of the same smell, it will spur your brain to pull of the information from your study sessions. A good example would be to eat peppermints while you are studying the periodic table, and then eat the same peppermints during the exam. Be careful to only use this for one subject at a time, if you eat peppermints all day then it won’t form an association specifically to the periodic table.















Comments
0 comment