
views
Using TCPO (Multiple Colors)
Wear clothing to protect your eyes and skin. Some of the chemicals are carcinogenic and incredibly dangerous when used improperly. It should also be noted that these are expensive and often difficult to obtain chemicals, best bought in bulk. They are not conducive to only making one or two glow sticks. At the very least, you should have: Latex gloves Ventilated goggles (lab goggles) Long sleeves Face mask A clean, tidy work station Clean glass tubes or containers with matching lids.

Start with 10mL of the solvent Diethyl Phthalate (DP). This is your base and makes up the majority of the liquid in your glow sticks. It will hold the chemicals that actually glow and amplify them. Start with 10mL of DP, but know that you can double or half this for bigger or smaller sticks. This looks like water, but the chemical reaction necessary for glowing won't work in water You may have to dig around a bit online to find these chemicals. Chemistry giants Alfa Aesar and Sigma Aldrich are two great resources. If you double the solvent, then you should double the rest of the ingredients below.
Add 3 mg of your chosen fluorescent dye to add color. You cannot use normal or additive dyes; make sure you're using fluorescent dyes. These dyes will not be the same color un-mixed as they are when they glow, so trust the guide given below to make your colors. There are a variety of different dyes you can use, depending on the color you want: 9,10-bis(phenylethynyl)anthracene for green Rubrene for yellow 9,10-diphenylanthracene for blue Rhodamine B for red (note: rhodamine decays quickly, meaning reds tend to fade fast). Mix half yellow, half blue to create white.

Add 50 mg of TCPO to the dyed mixture. TCPO stands for bis (2,4,6-trichlorophenyl) oxalate, which you may need to know to purchase it. It's quite expensive to buy, but you can make it fairly cheaply if, again, you're experienced and competent around chemicals. Otherwise, making it is not advised. The TCPO is used instead of luminol in this method -- it's the component that makes the mixture glow and it lasts for hours. TCPO is incredibly carcinogenic, and should be handled with care. Never inhale TCPO.
Add 100 mg of sodium acetate to the mixture. If you don't have sodium acetate, an even mixture of sodium bicarbonate (baking soda) and sodium salicylic can effectively be used instead. When added, put the lid on and shake the bottle well.
Finally, add 3 milliliters (0.10 fl oz) of 3% hydrogen peroxide to the mixture. It's important to do this step last, as it creates the chemical reaction. Put the lid back on, shake it up well, and turn off the lights. You should have a quite impressive stick/tube/container glowing ferociously in front of you. The hydrogen peroxide is an activator, not part of the reaction, so you won't need much. When you buy glow sticks, the "crack" noise to activate them is breaking a little glass vial of hydrogen peroxide.
Add larger quantities of TCPO and Sodium Acetate to make the reaction go on for longer. If you so desire, mess around with the recipe to see what warrants the best results. This reaction works because TCPO and Sodium Acetate release energy when combined, as they start to decay. This energy is picked up by the florescent dyes, which can convert energy into light. More of each leads to more energy and a longer reaction.
Using Luminol

Put on protective glasses. In addition, wear gloves to protect your skin. It's also a good idea to not wear your Sunday best. Throw on some old clothes or put a smock over clothes you want protected. Some of this stuff is dangerous -- this experiment is not meant for children! Listen up, kids: You'll be working with a solution that's near a 12 on the pH scale. That basically means don't swallow it, don't put it in your eyes, don't bathe in it, and don't really expose yourself to it directly at all. Got it? Moving on.
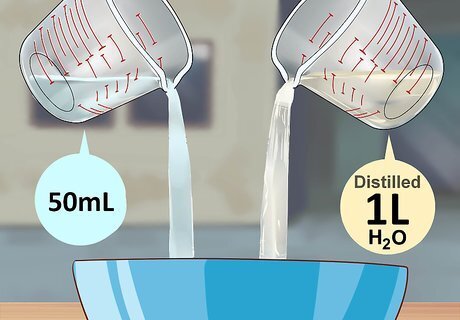
Combine 50 milliliters of 3% hydrogen peroxide and a liter of distilled water in a mixing bowl. A ceramic bowl will work best, but plastic works too. Use funnels, measuring tubes, and basters to keep everything well-measured and well away from you. Hydrogen peroxide is used to replace the luminol's nitrogen atoms with oxygen. When that happens, all the substances create a rave and start partying and electrons fly everywhere and what results? The glow.

Mix .2 grams of luminol, 4 grams of sodium carbonate, 24 grams of sodium bicarbonate, .4 grams (0.01 oz) of copper sulfate, .5 grams (0.02 oz) of ammonium carbonate and 1 liter (0.3 US gal) of distilled water in a second bowl. It is important not to touch the luminol. Use a funnel to make everything safe and easy. Unfortunately, these hazardous chemicals will not float freely in mid-air like this graphic suggests. Yep, unless you're a coroner or some sort of crazy spy/criminologist you probably don't have this stuff lying around the house (hopefully not...). If you're dead set on starting your own glowstick business (worse ideas exist), try websites like Alfa Aesar or Sigma Aldrich for supplies. Mix everything well. Don't use your hands -- use a metal or plastic utensil of some sort.
Clean the containers and dry them thoroughly. It's important to use sanitary, clean tubes for your glowsticks. The last thing you want is other substances interacting with the reactions you're depending on to make the substances glow.
Set the correct lid next to each container. This enables you to seal the containers quickly after filling. It's not like the glow will get up and run away from you, but still.
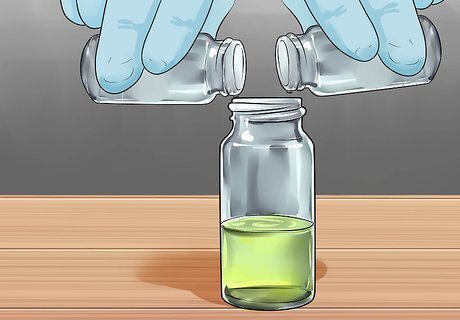
Combine equal amounts of the first and second solution in the container and close the bottles. Shake them up once the lids are on tightly. Then turn off the lights! If it's not already glowing, something went wrong. Do over!

Watch as the chemical compound creates a colorful glow. Take your glowsticks to the party and charge your friends loads of money for them! But act quickly...the glow won't last very long. Expectations crushed? Method one to the rescue! The reaction that the luminol and hydrogen peroxide creates doesn't last long at all -- maybe a couple of minutes. For something that lasts hours, go to the next method (which is a lot easier to facilitate if you have access to a laboratory, but it's still worth mentioning).














Comments
0 comment