
views
X
Research source
Insert a water bottle, cool it to 17 °F (−8 °C), and then tap it against a hard surface to freeze the water.
Creating a Salt and Ice Water Mixture
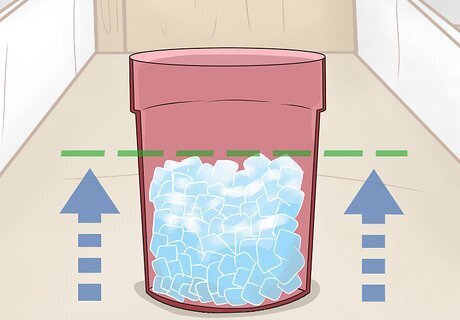
Pack your bucket or cooler about half full of ice. Your bucket or cooler should be large enough to hold both bottles of water without them touching each other. It should also be tall enough for the salt and ice water mixture to cover the water in your bottles. Check if your bucket is large enough by putting both water bottles in it while it’s still empty. You’ll be adding the water bottles after you create the salt and ice water mixture.
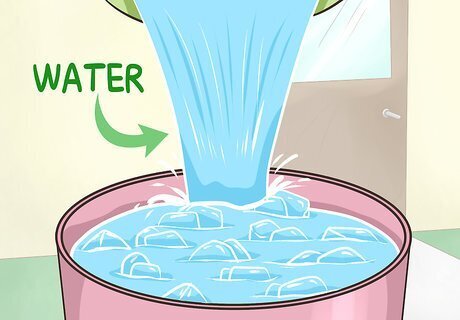
Pour in enough water to allow the ice cubes to move. Slowly add tap water from a sink or faucet. You should add enough so that the ice cubes can move more easily, but they should not float to the surface of the water. You should have more ice than water in your bucket or cooler.
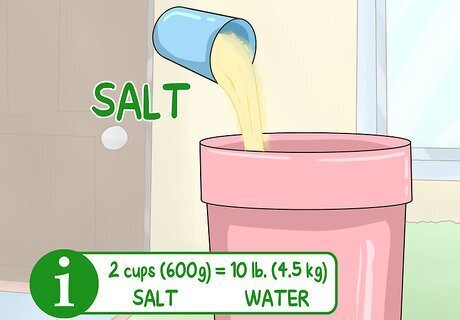
Add 2 cups (600 g) of rock salt for every 10 lb (4.5 kg) of ice. Mix the rock salt into the ice water slowly using a large spoon or ladle. Mixing should be easy enough with the amount of water that you added to your ice.
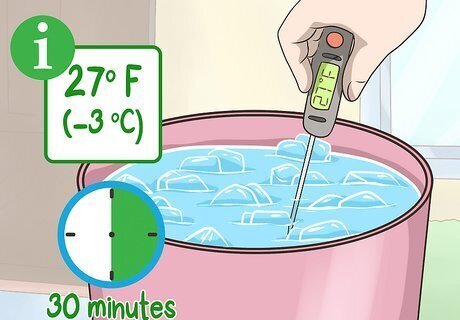
Allow the temperature to reach 27 °F (−3 °C). Use your thermometer to check the temperature after about 30 minutes. It should be below the freezing point of water. If it hasn’t dropped below that temperature, add 1 cup (300 g) of salt and mix.
Freezing Your Water Bottles

Place the water bottles carefully in the ice water. Once your mixture is ready, add the water bottles. Make sure they don’t touch each other, which could make them freeze early. You can use all kinds of water: purified, distilled, spring, or deionized. Don’t use glass bottles, which can burst. Don't use tap water. Ice crystals can form around impurities in tap water, which will ruin the supercooling process.
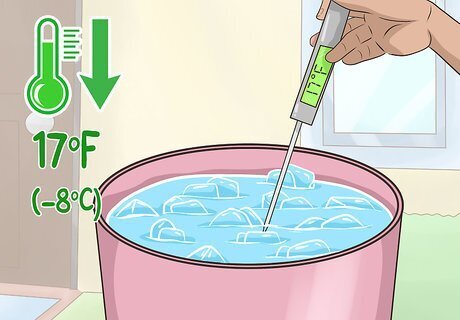
Allow the temperature to drop to 17 °F (−8 °C). Monitor your mixture with a thermometer over the next half hour to an hour until it reaches this temperature. Make sure the water in the bottles has not frozen. If the water has frozen, let the bottle thaw before trying again from the beginning.
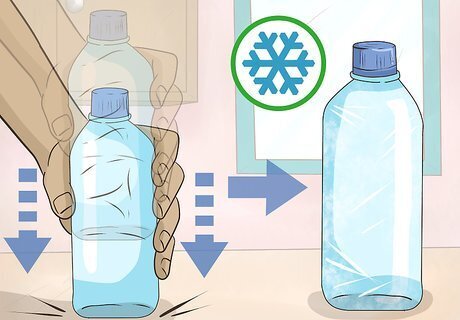
Tap a water bottle firmly against a hard surface. You can hit the ground, kitchen counter, or table. Ice crystals will form at the top of the bottle and creep down to the bottom. Open the cap of your second water bottle, and it will freeze the same way without being tapped. The motion of unscrewing the cap on the second bottle is enough to set off the ice crystals. If the water doesn’t freeze, tap it more firmly. If that does not work, return it to the ice water mixture and let it cool for another 30 minutes before trying again.


















Comments
0 comment