views
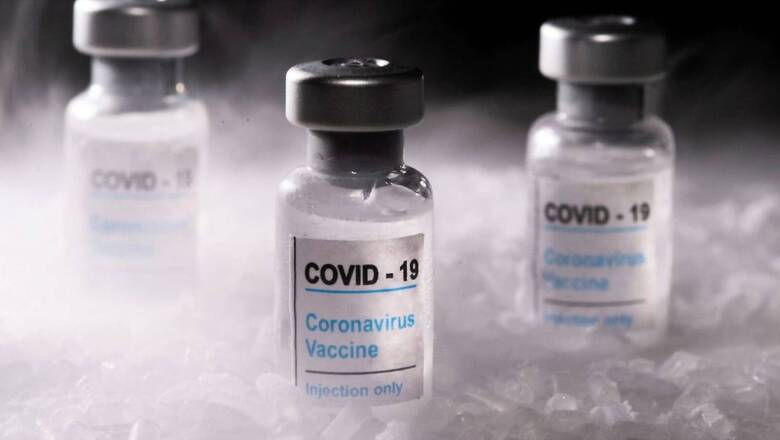
Bharat Biotech has been given an emergency use authorisation by India’s drug regulator, the Drug Controller General of India (DCGI), for its intranasal Covid-19 vaccine, Union Health Minister Dr Mansukh Mandaviya tweeted on Tuesday. The vaccine can now be administered to those above 18 years of age.
Mandaviya said the development would further provide a fillip to “our collective fight” against the Covid-19 pandemic.
Big Boost to India's Fight Against COVID-19!Bharat Biotech's ChAd36-SARS-CoV-S COVID-19 (Chimpanzee Adenovirus Vectored) recombinant nasal vaccine approved by @CDSCO_INDIA_INF for primary immunization against COVID-19 in 18+ age group for restricted use in emergency situation.
— Dr Mansukh Mandaviya (@mansukhmandviya) September 6, 2022
The health minister emphasised that India has harnessed its science, research and development (R&D), and human resources in the fight against the pandemic under Prime Minister Narendra Modi’s leadership. “With the science-driven approach & Sabka Prayas, we will defeat Covid-19,” Mandaviya also said.
The Hyderabad-based firm completed clinical trials of the nasal vaccine with about 4,000 volunteers and there is no side effect or adverse reaction reported so far, company sources had said.
Last month, the Hyderabad-based Bharat Biotech International Limited (BBIL) had said its Covid-19 intranasal vaccine (BBV154) has proven to be safe, well-tolerated, and immunogenic in subjects in controlled clinical trials phase III. The trials were conducted with the help of about 4,000 volunteers.
The vaccine candidate was evaluated earlier in phase I and II clinical trials with successful results.
The vaccine, named BBV154, has been specifically formulated to allow intranasal delivery. In addition, the nasal delivery system has been designed and developed to be cost-effective in low and middle-income countries, a press release from the vaccine maker said.
BBV154 was developed in partnership with Washington University St Louis, which had designed and developed the recombinant adenoviral vectored constructs and evaluated them in preclinical studies for efficacy. Product development related to pre-clinical safety evaluation, large-scale manufacturing scale-up, formulation, and delivery device development, including human clinical trials, was conducted by Bharat Biotech.
Read all the Latest News India and Breaking News here
















Comments
0 comment