views
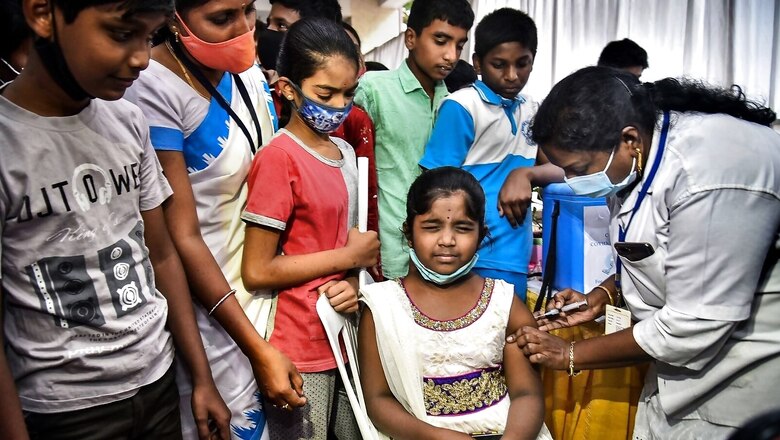
The Subject Expert Committee (SEC) of DCGI – India’s drug regulator – on Thursday recommended the use of Biological E’s Corbevax in children aged 5-12 years as reported by News18 earlier. The panel met at noon to discuss data and the use of the vaccine in children in that age bracket.
The recommendations have now been sent to the Drug Controller General of India by the SEC. A nod by the DCGI is now awaited before the Union Health Ministry gives a final go-ahead.
Corbevax is currently being administered to children in the age group 12-14 years.
India is currently administering two Covid-19 vaccines to children above 12 years of age. In phase one of children’s vaccinations in India – which started on January 3 this year – Covid vaccination was announced for children between 15-18 years of age, which was later expanded to cover kids aged above 12 years from March 16.
Bharat Biotech’s Covaxin is currently being administered to children in the age group of 15-18 years in both private and government vaccination centres, while Corbevax is being administered only in government centres in the age group of 12-14 years.
Corbevax vaccine, which is India’s indigenously developed Receptor Binding Domain (RBD) or protein sub-unit vaccine against Covid-19, uses similar technology that has been used for decades to make hepatitis B vaccines. The vaccine is administered through an intramuscular route with a two-dose scheduled 28 days apart.
Read all the Latest News India and Breaking News here



















Comments
0 comment