views
The Subject Expert Committee (SEC) has recommended grant of market authorisation to Bharat Biotech’s Covaxin for children in the 2-18 age group, but with certain riders. The SEC has sent recommendations to the Drugs Controller General of India (DCGI), which is likely to soon give a go-ahead.
Hyderabad-based Bharat Biotech had completed Phase-2 and Phase-3 trials of Covaxin on children below 18 years of age in September and submitted the trial data to the DCGI at the start of this month.
The authorisation, however, is subject to four conditions. One, the firm should continue the study as per the approved clinical trial protocol; two, the firm should provide updated Prescribing Information/Package Insert (PI), Summary of Product Characteristics (SmPC) and Factsheet; third, the firm should submit safety data, including the data on AEFI and AESI, with due analysis, every 15 days for the first two months and monthly thereafter and also as per requirement of New Drugs & Clinical Trials Rules, 2019; and last is that the firm should submit risk management plan. Further all other conditions of the earlier approval in the age group of ≥ 18 years shall remain same.
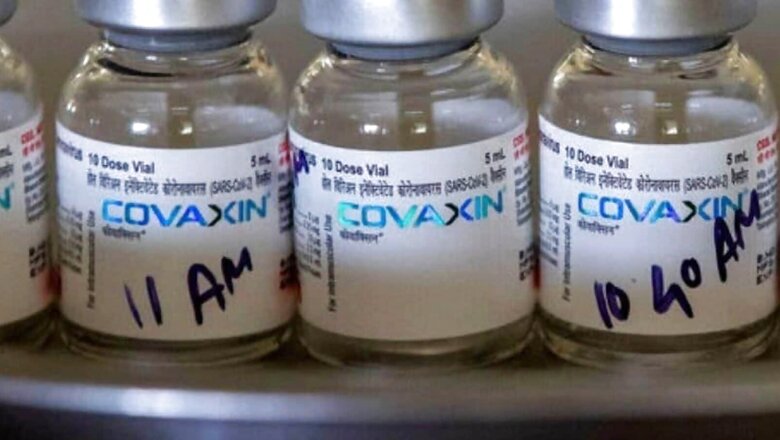
Unlike the Covaxin for adults and other Covid-19 vaccines currently being administered that are available in multi-dose vials, the paediatric Covaxin will use a pre-filled syringe or PFS mechanism. Pre-filled syringes are known to have a high-level accuracy, which makes them safer to use.
The vaccine dosage for children would be 0.5ml, the same as for adults. However, when administering vaccines to children, the accuracy of the dose is crucial.
Administering vaccines from vials to syringes can at times be inaccurate, resulting in the dose being less or more than 0.5ml, which is the recommended dose.
In the case of children as young as 2 years, a high dosage could be problematic and therefore, a PFS mechanism has been sought for the paediatric vaccine, a source in the know told CNN-News18. One pre-filled 0.5 ml of vaccine is to be used once and discarded.
Zydus Cadila’s three-dose needle-free vaccine ZyCov-D was approved in August for emergency use and can be given to both adults and adolescents aged 12 and above. The vaccine, however, is yet to be made available on the market.
Serum Institute of India (SII) has also recently started conducting clinical trials in 7-11 age group for their Novavax vaccine, known as Covovax in India.
Read all the Latest News , Breaking News and IPL 2022 Live Updates here.


















Comments
0 comment