views
Building the Fuel Cell
Gather all of the necessary materials. To build a simple household fuel cell, you will need 12 inches (30 cm) of platinum or platinum-coated wire, a popsicle stick, a 9-volt battery and battery clip, clear tape, a glass of water, table salt (optional), a thin metal rod, and a volt meter. A 9-volt battery and battery clip can be purchased at an electronics or hardware store.
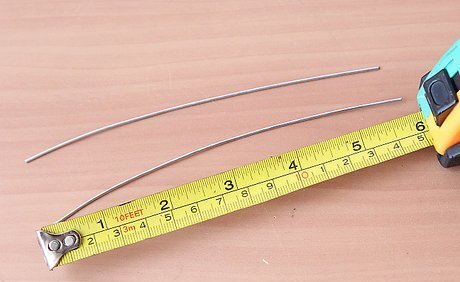
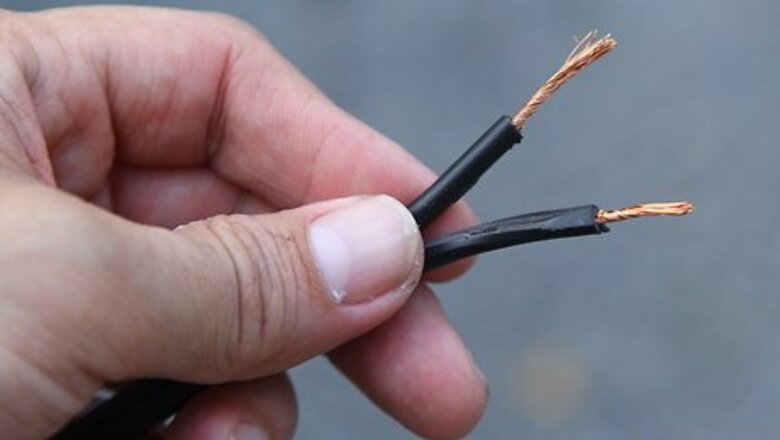
Cut two 6-inch (15-centimeter) strips of platinum or platinum-coated wire. You will need to purchase this wire from an electronics supply store, as platinum isn't used for common wiring purposes. The platinum serves as the catalyst for this reaction. Platinum wires are recommended because other substances, such as copper, will react with the oxygen or the salt to pollute your solution with the products of this reaction. High quality stainless steel can also be used as it will not react as readily.

Wind each wire strip around a thin metal rod to shape it into a spring. These two springs will serve as the fuel cell's electrodes. Take the end of the wire and wrap it tightly around your shaping rod to form a coil. Remove the first wire from the shaping rod and wrap the second wire. The shaping rod can be a nail, pick, wire coat hanger or lead on a battery tester.

Cut the leads from a 9-volt battery clip in half. Using a wire cutter, snip both of the wires attached to the clip in half and strip the insulation off the leads. This will leave bare wires that you can attach to your electrode coils. Using the stripping portion of the wire cutters, strip the insulation off of one end of the cut wires. Only strip the ends of the leads you cut off the battery clip. Make sure that you have parental supervision while you're cutting the wires.
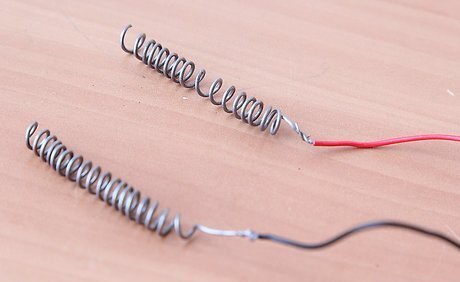
Attach the exposed wire ends to the electrode coils. Attaching the wire leads to the electrodes allows you to hook up your power source (the battery via the battery clip) and the voltmeter for reading how much electricity the fuel cell is producing. Twist the red battery clip lead and the red clipped wire lead around the top end of one of the coils, leaving most of the coil free. Twist the black battery clip lead and black clipped wire lead around the top end of the remaining coil.

Tape the electrodes to a popsicle stick or dowel rod. The popsicle stick needs to be longer than the mouth of the container holding the water so it can rest on top. Tape the electrodes so that they hang down, away from the stick and can be easily submerged into the water. You can use clear plastic tape or electrical tape. It doesn’t matter as long as the electrodes are firmly attached to the stick.

Fill the glass with tap water or salt water. In order to get a good reaction, the water solution needs electrolytes. Distilled water does not work well for this because there are no impurities to serve as electrolytes. Salt and baking soda dissolved in water serve as good electrolytes for the reaction. Regular tap water has impurities in it such as minerals that can serve as electrolytes if you don’t have any salt handy. Add a tablespoon of salt or baking soda for each cup of water. Stir until fully dissolved.

Lay the stick over the mouth of a glass of water. The coil electrodes should be submerged in the water for most of their length, except where they're connected to the wires from the battery clip. Only the platinum should be submerged. If necessary, tape the stick in place so the electrodes stay in the water.

Connect the wires coming from the electrodes to a voltmeter or LED bulb. The voltmeter is to show electric current created by the fuel cell once it's activated. Connect the red wire to the meter's positive terminal and the black wire to the negative terminal. You may see a small amount of voltage at this point, such as 0.01 volts, although the voltmeter should read zero at this point. You can also connect a small bulb, such as a flashlight bulb, or a light-emitting diode (LED).
Activating the Fuel Cell

Touch the 9-volt battery terminals to the battery clip for one to two seconds. The battery is needed only to send an initial current through the wire to cause the hydrogen in the water molecules touching the electrodes to separate from the oxygen, forming bubbles around the electrodes. This process is called electrolysis. Notice the bubbles forming around each electrode. One electrode has bubbles of hydrogen, while the other electrode has oxygen bubbles. The battery source does not need to be completely attached the clip, just touched to the battery terminals to begin the reaction.

Remove the battery. The battery is only needed to initiate the reaction. The separated hydrogen and oxygen will recombine into water, releasing the energy originally used to split them as electricity. The platinum in the electrode coils serves as a catalyst to speed the recombination of the hydrogen and oxygen into water molecules.

Read the output on the voltmeter. Initially, the output may be as great as two volts, but will decline as the hydrogen bubbles dissipate, quickly at first and then gradually as the last of the bubbles pop. A light bulb or LED may appear bright initially, but will quickly dim and then gradually fade away.



















Comments
0 comment